The official version of this document can be found via the PDF button.
The below content has been automatically generated from the original PDF and some formatting may have been lost, therefore it should not be relied upon to extract citations or propose amendments.
Heredity (2004) 92, 396 401
& 2004 Nature Publishing Group All rights reserved 0018-067X/04 $25.00
![]() www.nature.com/hdy
www.nature.com/hdy
Population genetic structure of and inbreeding in an insular cattle breed, the Jersey, and its implications for genetic resource management
L Chikhi1,2,5, B Goossens3,5, A Treanor4 and MW Bruford1,3
1Institute of Zoology, Regent s Park, London NW1 4RY, UK; 2Department of Biology, University College London, Darwin Building, London WC1E 6BT, UK; 3School of Biosciences, Cardiff University, PO Box 915, Cathays Park, Cardiff CF10 3TL, UK;4Ministry of Agriculture, States of Jersey, UK
The Jersey is a ubiquitous and successful breed of cattle that originates from the UK Channel Island of Jersey. While the breed has been exported extensively, no imports have taken place to the island since 1789, leading to a concern regarding possible losses of genetic diversity and increased inbreed- ing. We have conducted the rst large-scale genetic analysis
of the Jersey cattle using only samples from the island. A total of 223 cattle from all parishes except one were genotyped for 12 microsatellite loci. The average number of alleles per locus and expected heterozygosity were found to be comparatively high (nA ¼4, He ¼0.64) with respect to that observed in a number of continental breeds. Only breeds that have been upgraded and are therefore the result of admixture are clearly more variable than the Jersey. We also found a signi cant but limited amount of genetic differentia- tion between parishes (Fst ¼0.013), or even between farms
(Fst ¼0.035) despite an apparent lack of movement. This is con rmed by the application of two recent statistical methods. A Bayesian partition analysis shows that the most probable value of K, the number of possible hidden partitions,
is 1 (PB 0.98). K¼2 has a much lower probability ( PB 0.02) while other values are essentially zero. Similarly, we were able to show that there was no support for departure from panmixia other than due to population structure, and thus
that there is suf cient background gene ow across the island to overcome local drift. Overall, it appears that the current level of genetic diversity and its distribution within the island means it is unnecessary to import unrelated genetic material to the island for management purposes. Heredity (2004) 92, 396401, advance online publication, 10 March 2004; doi:10.1038/sj.hdy.6800433
Keywords: Jersey cattle; Island of Jersey; microsatellites; genetic diversity; inbreeding
Introduction
The characterisation of indigenous, economically impor- tant genetic resources in agriculture has become an issue of ever increasing importance, both for scienti c and ownership reasons. Most countries are increasingly aware that their ora and fauna may have unique and potentially valuable genetic attributes that may be revealed through the quanti cation of genetic distinc- tiveness and/or the demonstration of genetic unique- ness. While these notions are widely recognised among conservationists for natural populations or species, the case of breeds may be even more acute, due to the large amount of arti cial selection that has taken place and which has favoured the appearance of local and economically important adaptations (Whitlock, 1980; Alderson, 1994; Hall and Bradley, 1995).
Methods to quantify the distribution of genetic diversity within and among breeds have been applied to both indigenous and ubiquitous breeds of cattle. Microsatellite data have shown that signi cant levels of
Correspondence: L Chikhi, UMR 5174 Evolution et Diversite· Biologique, Bat. IV R3, Universite· Paul Sabatier, 118 Route de Narbonne, 31062 Toulouse Ce·dex 4, France. E-mail: chikhi@cict.fr
5The first two authors contributed equally.
Received 7 October 2002; accepted 16 January 2004; published online 10 March 2004
genetic differentiation may exist between European cattle breeds (MacHugh et al, 1994, 1998) or between herds within breeds (Blott et al, 1998b).
However, most genetic studies within cattle breeds to
date have examined only limited samples within speci c breeds. One exception is the study of Blott et al (1998b) who examined genetic differentiation for Hereford
populations from different continents.
Here, we present the rst comprehensive study of the genetic structure of a cattle breed within its entire native range using the indigenous Jersey Island breed. While the Jersey has now become ubiquitous it exhibits a very signi cant difference with the Hereford. The Island of Jersey
has been isolated from any imports of cattle from the rest of
the world, including England, since 1789. We can, therefore,
rule out the effects of recent introgression from other breeds
as a source of genetic variation in this case. Moreover, full pedigree records have been kept for about 250 years.
We examined the genetic diversity and structure of the Jersey Island breed, comparing our estimates to those obtained for other breeds. We also examine a long- standing problem in livestock genetic diversity by addressing the effects of differing sampling regimes (across the entire island, within parishes, and farms) on estimates of genetic diversity. By doing so, we also tested
for evidence of inbreeding and assessed the correlation between geographic differentiation and geographic separation among parishes.
Materials and methods
Sampling
Cattle were sampled across the island in order to obtain individuals originating from as many parishes as possible. We obtained 223 samples from 11 out of the 12 parishes (Figure 1 in Supplementary Data), represent- ing more than 5% of the ca 4000 cattle present on the island (Table 1). These samples also included 24 additional individuals from various additional locations
on the island. This sample comprised a random mixture
of cattle from the island and was used for comparisons in some of the preliminary analyses. The sample sizes varied among parishes: St John (n ¼25), St Peter (n ¼24), Grouville (n ¼9), Trinity (n ¼19), St Mary (n ¼18), St Brelade (n ¼11), St Saviour (n ¼39), St Martin (n ¼23), St Clement (n ¼4), St Helier (n ¼16), St Ouen (n ¼11) (see Figure 1 in Supplementary Data and Table 1). For all parishes, except St Helier, Trinity and St Clement, cattle came from more than one farm (Table 1 in Supplemen- tary data). Hair samples were plucked from each individual and stored in paper envelopes at room temperature.
 a
a
0.9 0.8 0.7 0.6
DNA extraction
DNA was extracted from at least 10 plucked hairs per individual following the Chelex-100 procedure described
in Walsh et al (1991) with speci c details in Goossens et al (1998). In total, 400 ml samples were incubated at 561C for 56h, and then put in a boiling water bath for 8min. In
all, 2.5 ml of each extract were added as template in each PCR reaction.
Microsatellite analysis
A total of 12 microsatellite loci (HAUT27, HEL5, BM1314, BM1818, BM2113, INRA005, INRA063, ILSTS006, ETH10, ETH225, TGLA122, and TGLA227 (Steffen et al, 1993; Vaiman et al, 1994; MacHugh et al, 1998)) were ampli ed with GibcoTaqs in a Perkin-Elmer Gene Amp PCR System 9600. One primer from each pair was synthesized
with a uorescent dye, FAM, HEX or TET, on the 5[0] end. Ampli cation of the loci was carried out in 12.5 ml reactions (10mM Tris-HCl (pH 9.0), 200mM (NH4)2SO4, 50mMeachdNTP,1.5mMMgCl2,5ngBSA,0.1UAmplitaqs Gold DNA polymerase (Perkin-Elmer), 0.5 mM (for FAM) or 0.75 mM (for TET) or 1 mM (for HEX) uorescent primer, same concentration for the non uorescent primer). Thermocycling conditions were as follows: initial denaturation at 931C for 30 followed by 40 cycles
of 15s at 951C, 30s at annealing temperatures (which varied from 50 to 601C depending on the locus, see Supplementary Data of Table 2), extension of 30s to 1min at 721C for 300 to 10), and a nal extension at 721C
for 20. All PCR products were separated on an acryl- amide gel using an ABI PRISM 377 DNA sequencer. Gels were analysed using the GeneScan Analysis 2.0t and Genotyper 1.1t software.
Data analysis
Table 1 Measures of genetic diversity
1 2 3 4 5 6 7 8 9 10 11 12 13 St John St Peter Grouville Trinity St Mary St Brelade St Saviour St Martin St Clement St Helier St Ouen moved Var. Loc. | Total | ||||||||||
n 25 24 9 HAUT27 5 4 2 He 0.6098 0.4087 0.4248 Ho 0.5200 0.4167 0.3333 HEL5 5 5 5 He 0.6580 0.5984 0.7582 Ho 0.5600 0.6250 0.7778 BM1314 4 5 4 He 0.6073 0.6215 0.5752 Ho 0.6400 0.4167 0.3333 BM1818 5 4 4 He 0.5967 0.6144 0.6013 Ho 0.5200 0.6250 0.5556 BM2113 4 4 3 He 0.6588 0.6285 0.6601 Ho 0.7600 0.6250 0.6667 INRA005 3 3 3 He 0.6376 0.6463 0.5817 Ho 0.4800 0.6250 0.6667 INRA063 3 3 3 He 0.5641 0.5310 0.5817 Ho 0.5200 0.5417 0.3333 ILSTS006 5 6 4 He 0.5853 0.6817 0.7647 Ho 0.6000 0.7083 0.6667 ETH10 5 5 5 He 0.6049 0.6250 0.6732 Ho 0.6400 0.7917 0.7778 ETH225 3 3 3 He 0.6016 0.4406 0.6275 Ho 0.6400 0.4583 0.5556 TGLA122 3 4 4 He 0.6033 0.7012 0.7582 Ho 0.6400 0.7917 0.7778 TGLA227 8 8 6 He 0.8253 0.8209 0.8497 Ho 0.9200 0.8333 0.6667 Total nA 4.42 4.50 3.83 He 0.6294 0.6099 0.6547 Ho 0.6200 0.6215 0.5926 | 19 18 4 3 0.4723 0.4889 0.5789 0.6111 5 4 0.6700 0.6937 0.6316 0.8333 5 4 0.6302 0.6238 0.8421 0.6667 5 5 0.6885 0.7333 0.6316 0.5556 4 4 0.6074 0.7413 0.5789 0.7778 3 3 0.6529 0.6524 0.6316 0.6667 3 3 0.5220 0.6238 0.5789 0.5556 6 4 0.8151 0.5730 0.7895 0.8333 4 4 0.7070 0.5762 0.7368 0.5000 3 3 0.6188 0.6175 0.6842 0.5556 5 4 0.6913 0.6175 0.7368 0.6667 8 7 0.8535 0.7905 0.9474 0.8889 4.58 4.00 0.6607 0.6443 0.6974 0.6759 | 11 3 0.4502 0.3636 3 0.3247 0.3636 2 0.4156 0.5455 5 0.6147 0.6364 4 0.6537 0.7273 3 0.6017 0.7273 3 0.5411 0.4545 5 0.7359 0.6364 4 0.6710 0.8182 3 0.5541 0.7273 4 0.7446 0.7273 7 0.7965 0.7273 3.83 0.5920 0.6212 | 39 3 0.4632 0.5385 5 0.6863 0.6667 4 0.5288 0.5897 6 0.6926 0.5128 4 0.6750 0.6410 3 0.6111 0.6410 3 0.6264 0.5385 7 0.7160 0.6410 5 0.6217 0.6923 3 0.6410 0.6923 5 0.6537 0.7179 8 0.7692 0.7692 4.67 0.6404 0.6368 | 23 3 0.5343 0.3478 4 0.6077 0.5217 5 0.5720 0.5217 0.6271 0.6087 4 0.5729 0.5652 3 0.5807 0.6087 4 0.6686 0.6087 6 0.7304 0.6957 5 0.5411 0.6522 3 0.5053 0.3043 4 0.6531 0.6957 8 0.8164 0.6522 4.42 0.6175 0.5652 | 4 3 0.6071 0.2500 4 0.7500 0.7500 4 0.6429 0.7500 4 0.4286 0.5000 3 0.7500 0.7500 2 0.4286 0.0000 3 0.4643 0.5000 3 0.6786 0.5000 4 0.7857 0.7500 3 0.7143 1.0000 4 0.8214 0.7500 6 0.9286 1.0000 3.42 0.6667 0.6250 | 16 11 14 4 4 5 0.5786 0.5931 0.5635 0.7500 0.4545 0.6429 4 4 4 0.7177 0.6623 0.5503 0.9375 0.6364 0.5000 3 2 3 0.5222 0.5195 0.6111 0.6875 0.5455 0.4286 3 2 3 0.7802 0.7749 0.7354 0.6250 0.6364 0.5000 4 4 4 0.7298 0.7229 0.4286 0.8125 0.6364 0.5000 3 3 3 0.5867 0.6710 0.6376 0.8125 0.7273 0.5714 3 4 3 0.4940 0.6537 0.3624 0.5000 0.6364 0.4286 5 5 5 0.7977 0.6883 0.7302 0.6667 0.5455 0.6429 4 4 5 0.6149 0.6407 0.5979 0.7500 0.4545 0.7143 3 3 3 0.5988 0.6797 0.5952 0.5625 0.7273 0.8571 4 3 4 0.6673 0.6537 0.5212 0.7500 0.8182 0.5000 9 5 8 0.8831 0.6277 0.8783 0.7500 0.6364 0.9286 4.25 3.75 4.42 0.6643 0.6573 0.6010 0.7170 0.6212 0.6012 | 10 3 0.4263 0.5000 5 0.5579 0.7000 4 0.6211 0.7000 4 0.7947 0.8000 4 0.6579 0.5000 3 0.6789 0.5000 3 0.6368 0.7000 3 0.6737 0.8000 4 0.7263 0.8000 3 0.4684 0.6000 3 0.6789 0.8000 6 0.6211 0.6000 4.00 0.6285 0.6667 | 223 5 0.502 0.506 6 0.638 0.645 6 0.580 0.587 6 0.715 0.583 5 0.653 0.654 3 0.628 0.618 5 0.572 0.538 7 0.706 0.680 5 0.624 0.690 3 0.598 0.609 5 0.657 0.713 11 0.837 0.798 0.643 0.635 | |||
n is the sample size, H and H are the observed and expected heterozygosities, and o e | n is the mean number of alleles. A |
|
| ||||||||
Table 2 Pairwise Fst values between parishes |
|
|
| ||||||||
| St John St Peter Grouville Trinity St Mary St Brelade St Saviour St Martin St Clement St Helier St Ouen moved Var. loc. | ||||||||||
St John St Peter Grouville Trinity St Mary St Brelade St Saviour St Martin St Clement St Helier St Ouen Moved Var. Loc. | 0 NS NS *** NS * NS NS NS ** NS NS * | 0.00989 0.00074 0.03298 0.00309 0.02024 0 0.00336 0.03443 0.00965 0.01059 NS 0 0.02393 0.00194 0.00785 *** * 0 0.03176 0.05190 NS NS *** 0 0.03767 NS NS ** ** 0 ** NS *** NS NS NS NS *** NS NS NS NS * NS NS ** NS NS NS ** NS NS NS NS NS * * NS NS * * * * NS * | 0.00561 0.00110 0.03242 0.02281 0.00774 0.01245 0.02535 0.01455 0.00531 0.03118 0.03112 0.01561 0.02428 0.02502 0.00496 0.00371 0.00894 0.00179 0.00665 0.01516 0.03188 0.02912 0.03458 0.05077 0.01234 0.01273 0.01116 0.02767 0.00909 0.01252 0.01492 0.01178 0.00293 0.02700 0.01733 0.01156 0.00911 0.01360 0.03792 0.01656 0.03622 0.03013 0 0.00151 0.02188 0.00945 0.00621 0.02000 0.01749 NS 0 0.04078 0.02104 0.00237 0.01354 0.02770 NS NS 0 0.02175 0.02027 0.05783 0.07176 NS * NS 0 0.01774 0.02433 0.02153 NS NS NS NS 0 0.01015 0.02016 * NS * * NS 0 0.04471 * * ** NS NS ** 0 | ||||||||
Signi cant values are in bold. NS, not signi cant; *** Po0.001; ** 0.001pPo0.01; *0.01pPo0.05.
genetic and geographic distances between parishes or We also used a recent method developed by Overall farms. and Nichols (2001) to separate the effect of substructure All these computations were performed using GENETIX (Wahlund effect) and consanguinity in apparent
4.0 (available at http://www.univ-montp2.fr/ B genetix/ inbreeding as measured by positive F is. The method genetix.htm). generates the joint likelihood distribution of y (Weir and
Cockerham s measure of Fst) and C, the proportion of the population that is consanguineous to a certain level and therefore allows us to nd the maximum likelihood values for both parameters. The proportion C depends
on how consanguineous individuals are, and the calculation can be done including many different levels
of consanguinity at once. However, given the low levels
of inbreeding observed, the analysis was carried out for a value of 1/16 corresponding to offspring of rst cousins
(A Overall, pers. comm.).
Dawson and Belkhir (2001) recently developed a method that detects and tests for partition within any genetic sample without prior information on the origin of individuals. This method allows the detection of up to 12 partitions (or subgroups). A Monte Carlo Markov Chain (MCMC) approach using the MetropolisHastings algo- rithm is taken to explore the parameter space de ned by (i) K, the number of possible partitions, and (ii) the distribution of individuals in the K partitions. During this process the likelihood of the data is estimated for the different K values and possible assignments of indivi- duals to the Kpartitions. The theory shows that when the chain reaches equilibrium the different values of K have been sampled in proportion to their probability of generating the data. It therefore becomes possible to estimate the posterior probability distribution of K and jointly estimate the posterior probability that any two individuals belong to the same partition. Avalue of K¼1 thus means that no hidden substructure is detected in the data set. The advantage of this method is that it does not depend on the units de ned by our sampling strategy. It tries to recover any hidden partition in the data. The maximum value of K has to be speci ed beforehand. Given that the time of the analysis increases very quickly with increasing K values, the maximum was set to K¼8 and proved to be large enough.
Results and discussion
Variability and isolation
In total, 67 alleles were detected at the 12 loci surveyed among the 223 Jersey cattle, giving a mean number of approximately four alleles per locus (Table 1). This number varied across loci with only three alleles at INRA005 and ETH225 and 11 alleles at TGLA227. The average number of alleles per sample per locus exhibited some variation with 3.42 alleles in St Clement (which was
the smallest sample with only four cattle) and 4.67 in St Saviour. He values varied between 0.32 in St Brelade (locus HEL5) and 0.93 in St Clement (locus TGLA227). The average He values for each sample varied between
0.59 in St Brelade and 0.67 in St Clement, with a global average of 0.64.
A previous genetic study found that the Jersey cattle seemed to exhibit a reduced genetic diversity when compared to continental breeds (MacHugh et al, 1994). These authors found that, using 12 microsatellites, the British Isles breeds only had 3.3 alleles per locus compared to 4.3 in a number of continental breeds such
as the Charolais, Friesian and Simmental. A later study based on 20 loci (MacHugh et al, 1998) con rmed the high level of genetic diversity of the Charolais and Friesian breeds.
While these results seem to conform to the expectation
that island breeds will have lower effective sizes and therefore exhibit lower He values, there are two important caveats in such comparisons. First, the large genetic diversity observed in Charolais and Friesian breeds appears to be explained by the introgression
of genes from a number of smaller breeds (MacHugh
et al, 1997). In other words, admixture events taking place in some herds, whether known or unrecorded,
may bias He estimations. Second, the loci used appear
to also in uence the apparent level of diversity.
This effect, which is related to ascertainment bias, is rarely acknowledged, probably because it is dif cult to assess, but appears to be substantial in the study of MacHugh et al (1994). Indeed all breeds, including the Jersey cattle, had a He between 0.403 and 0.488 whereas more recent studies (Peelman et al, 1998; Loftus et al, 1999; Martin-Burriel et al, 1999, Table 3) found He values mostly between 0.55 and 0.70. In fact, MacHugh et al s (1994) study was among the rst and used microsatellites linked to genes without prior polymorphism pre-screening.
Assuming that,with theexceptionof theMacHugh et al (1994) study, He values can be compared across pub- lished data, Jersey cattle appear to be just as variable as a number of continental breeds (Table 3). This assumption
can be tested by recalculating He values for the seven loci that are in common between our study and that of Loftus
et al (1999), for which we nd He ¼0.63 (the value found by these authors).
Our results thus indicate that (i) despite its isolation
and particular history the Island Jersey is far from being
the least variable of cattle breeds, (ii) more work is needed on the distribution of variability across loci.
Inbreeding and isolation
Overall, the average Fit across loci was positive and highly signi cant (Fit ¼0.013, Po0.001). While this might be interpreted as signi cant inbreeding at the island level we are able to show that a Wahlund effect is the most likely explanation. For instance, most Fis values were low within parishes and nonsigni cant (overall Fis ¼ 0.003, NS). The 11 signi cant Fis values did not show any local trend (see Supplementary Data of Table 2). The only parish that exhibited an overall signi cant Fis (Fis ¼0.086, Po0.05) was St Martin but the method of Overall and Nichols (2001) indicated that it was most likely due to substructure. At the farm level, average Fis values were negative but not signi cant (Fis ¼ 0.023, NS).
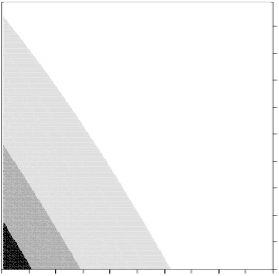
These results were con rmed by the Overall and Nichols (2001) method, which showed no apparent departure from random mating for most parishes. The maximum likelihood was zero for both parameters, as exhibited in Figure 1a for St Saviour. When all samples were analysed together there appeared to be a slight level of inbreeding. However, when we conditioned on the observed F stB 0.01 (see below), this effect disap- peared.
Allan (1987) showed, based on pedigree analysis, that the level of inbreeding observed in Island Jersey was relatively low (around 0.06) and he concluded that it was not potentially damaging to the breed. Our conclusions (based on the ability to separate the effect of inbreeding and substructure) con rm that there is no genetic threat
Table 3 Levels of variability among European cattle breeds in recent microsatellite studies
Nb loci | Breeds (He) | Ref. |
23 30 20 12 20 12 | Belgian Blue (0.65), Holstein Friesian (0.69), East Flemish (0.69), Red Pied (0.71) Menorquina (0.564), Fighting Bull (0.590), Pyrenean (0.617), Asturian Mountain (0.667), Nordwest Brown group (0.671), Asturian Lowland (0.681) No He values given. Hereford (0.403), Jersey (0.410), Angus (0.415), Simmental (0.432), Charolais (0.46), Friesian (0.488) N Dama (0.54), Hungarian Grey (0.62), Jersey (0.63), Ongole (0.64), Nellore (0.65), Charolais (0.66), Damascus (0.74), Turkish Grey (0.76), Anatolian Black (0.78), South Anatolian Red (0.78), East Anatolian Red (0.78), Egypt (0.78), Iraqi (0.78), Kurdi (0.79) Jersey (0.64) | Peelman et al (1998) Martin-Burriel et al (1999) MacHugh et al (1998) MacHugh et al (1994) Loftus et al (1999) This study |
currently posed by keeping the island demographically isolated. Importation of semen from American and Australian Jersey cattle to increase the gene pool for selection on the island and allow genetic improvement in the breed has been suggested. However, in the absence of molecular evidence for a genetically depauperate island population there seems little scienti c reason to ignore Allan s recommendations.
Differentiation between parishes and farms
The average Fst value was low but highly signi cant between parishes (Fst ¼0.016, Po0.001) explaining most
of the overall Fit. The pairwise Fst values ranged from 0.009 (ie 0) to 0.070 (Table 2). Removing the moved and Various locations samples, together with the St Clement sample, only resulted in minimal change (Fst ¼0.013, Po0.001). As expected, the genetic differentiation be- tween farms was higher than between parishes and is
also highly signi cant (Fst ¼0.035, Po0.001). The parti- tion analysis revealed a similar pattern. The posterior distribution for K, the number of partitions within the whole island, is shown in Figure 1b. It clearly indicates a high support for the whole island being considered as
one (more or less) panmictic population with only ca 2% support for a bipartition, and no support for K42. The result of the Mantel test was not signi cant (r ¼ 0.036, NS), indicating no signi cant correlation between geo- graphic and genetic distances.
The existence of signi cant genetic differentiation is
not surprising. The surprising result was the low Fst value. With a total population size of around 4000, cattle there are on average less than 400 cattle per parish. Assuming that drift occurred between parishes in the last
250 years (with approximately 5 years per generation),
Fst could have increased up to 0.06 (assuming that the effective size Ne ¼N). Given that Ne is likely to be considerably lower than the population size, we nd
that, if Ne ¼N/3, Fst ¼0.15, a value commonly found between breeds. Indeed, breed demographic histories are
complex and can generate high levels of genetic differentiation. In sheep we found Fst values as high as
0.09 between ocks of some European breeds, with a maximum of 0.12 between Soay ocks (Byrne et al, in press). Similarly, Blott et al (1998a) have found that levels of differentiation between recently separated Hereford cattle overlapped with those observed between the Hereford and other European breeds.
The observed Fst values are thus much lower than expected under pure drift and would have been reached in less than 50 and 20 years with Ne ¼N and Ne ¼N/3, respectively. Thus, our results strongly suggest that gene
ow across the island may be more widespread than we rst hypothesised. This conclusion, was, as we saw,
con rmed by the partition analysis. A mean Fst between parishes smaller than 0.02 indicates that, on average, each parish still retains more than 98% of the variability present on the whole island. At the farm level, the average is around 97%. While more similar work would
be needed to assess within-breed differentiation in continental breeds, these results suggest that sampling
only some areas of the island is likely to provide a good sample of the total genetic diversity of the Island Jersey.
Conclusion
Jersey Island cattle are unique in having been purpose- fully isolated from other UK and mainland European cattle populations for approximately 50 generations. For much of this period of isolation cattle are thought to have been mostly managed in relatively small groups on farms, mainly breeding with individuals located within a
short distance (for example, on farms within the same parish). This kind of demographic history was expected
to have generated (i) reduced diversity compared to breeds from the continent or mainland UK (eg Frank- ham, 1997), (ii) medium to high Fst values perhaps associated with (iii) some isolation by distance. While the extent of such effects was not easy to predict a priori, our results suggest that none is clearly detectable. Despite the increasing worries of farmers that inbreeding was accumulating across the island, our results suggest that
the Jersey Island cattle is just as variable as many other breeds. The level of inbreeding is low and does not appear to justify imports of semen from other Jersey populations.
While imports could indeed bring new alleles or genotypes, it is far from clear whether it is really necessary. Our recommendation of keeping the island isolated also draws from the experience of the Hereford cattle, another ubiquitous breed with huge success outside its area of origin. Blott et al (1998b) have shown that, for this British breed, imports from Canadian populations with higher performance have negatively affected the genetic diversity of the Hereford in the British Isles. The reason for this is that as soon as imports
are possible, the risk exists that farmers will tend to import semen offered from the same sires, potentially reducing the gene pool for future generations.
However, breeds can potentially suffer similar demo- graphic and genetic problems to threatened species and other small populations: genetic drift can arise rapidly under conditions of intense selection and arti cial insemination.
The level of genetic diversity observed in a breed is the result of a number of complex demographic factors and current or recent population size is not the only one of these. Indeed, the Hereford, Jersey and Holstein Friesian are all ubiquitous breeds but appear to exhibit different levels of polymorphism (Table 3). Similarly, the Belgian Blue has the largest population size of four Belgian breeds studied (Peelman et al, 1998), yet it had the lowest He value. This was explained by the fact that the Belgian Blue is a closed population whereas the other Belgian breeds had been graded up, resulting in the incorpora- tion of new alleles and an increase in genetic variability. One recommendation would also be that cattle such as the Jersey could serve as interesting comparisons with threatened species. The conservation genetics literature rarely uses information from such breeds and we hope that the present study might provide important com- parative data. Just as it is true for a number of protected species, the future genetic status of the Jersey Island cattle remains reliant on careful management.
Acknowledgements
LC was supported by MAFF contract OC9316B to MWB, the Institute of Zoology and Cardiff University. BG was supported by Leverhulme Grant F/390/U to MWB and JR de Ruiter and by DETR Darwin Initiative grant 09/016 to MWB. We thank Kate Byrne, Saffron Townsend, Rob Cuickshank and Jan de Ruiter for laboratory and sampling assistance and collaboration on MAFF con- tracts OCS9316 and OC9316B. Thanks also go to Dr GLH Alderson (Rare Breeds International), the Rare Breeds Survival Trust, Drs. Emma Hennessey and John Caygill (Chief Scientist s Group, MAFF), Dr Jean-Pierre Garnier (Meat Trade Advisor, MAFF) and Elizabeth Henson (Liaison Of cer, MAFF) for their support throughout this project. We are very grateful to Andy Overall for the inbreeding analysis and for comments on the results and especially to James Godfrey and David Hambrook (Jersey Society) and all the farmers from Jersey who allowed us to sample in their farms. This project is part of a programme in MWB s laboratory on genetic resource management in livestock, funded by DEFRA and the EC (www.cf.ac.uk/biosi/research/biodiversity/ staff/mb.html). Data are available upon request from the corresponding author.
References
Alderson L (1994). The Chance to Survive. Pilkington Press:
Yelvertoft.
Allan J (1987). To import the semen of superior tested sires or not to
import semen. The Albert Messervy Memorial Conference.
Blott SC, Williams JL, Haley CS (1998a). Genetic relation-
ships among European cattle breeds. Anim Genet 29: 273282.
Blott SC, Williams JL, Haley CS (1998b). Genetic variation
within the Hereford breed of cattle. Anim Genet 29: 202211. Byrne K, Chikhi L, Townsend SJ, Cruikshank R, Alderson L,
Bruford MW. Genetic diversity in phenotypically diverse
European sheep breeds: rarity and conservation. Mol Ecol (in
press).
Dawson KJ, Belkhir K (2001). A Bayesian approach to the
identi cation of panmictic populations and the assignment of individuals. Genet Res 78: 5977.
Frankham R (1997). Do island populations have less genetic
variation than mainland populations? Heredity 78: 311327. Goossens B, Waits LP, Taberlet P (1998). Plucked hair samples as
a source of DNA: reliability of dinucleotide microsatellite genotyping. Mol Ecol 7: 12371241.
Hall SJG, Bradley DG (1995). Conserving livestock breed
biodiversity. Trends Ecol Evol 10: 267270.
Loftus RT, Ertugrul O, Harba AH, El Barody MA, MacHugh
DE, Park SD, Bradley DG (1999). A microsatellite survey of cattle from a centre of origin: the Near East. Mol Ecol8: 2015 2022.
MacHugh DE, Loftus RT, Bradley DG, Sharp PM, Cunningham
P (1994). Microsatellite DNA variation within and among European cattle breeds. Proc Roy Soc London B Biol Sci256: 2531.
MacHugh DE, Loftus RT, Cunningham P, Bradley DG (1998).
Genetic structure of seven European cattle breeds assessed
using 20 microsatellite markers. Anim Genet 29: 333340. MacHugh DE, Shriver MD, Loftus RT, Cunningham P, Bradley
DG (1997). Microsatellite DNA variation and the evolution, domestication and phylogeography of taurine and zebu
cattle (Bos taurus and Bos indicus). Genetics 146: 10711086. Martin-Burriel I, Garcia-Muro E, Zaragoza P (1999). Genetic
diversity analysis of six Spanish native cattle breeds using
microsatellites. Anim Genet 30: 177182.
Nei M (1978). Estimation of heterozygosity and genetic distance
from a small number of individuals. Genetics 89: 583590. Nei M (1987). Molecular Evolutionary Genetics. Columbia Uni-
versity Press: New York.
Overall AD, Nichols RA (2001). A method for distinguishing
consanguinity and population substructure using multilocus genotype data. Mol Biol Evol18: 20482056.
Peelman LJ, Mortiaux F, Van Zeveren A, Dansercoer A,
Mommens G, Coopman F et al (1998). Evaluation of the genetic variability of 23 bovine microsatellite markers in four Belgian cattle breeds. Anim Genet 29: 161167.
Steffen P, Eggen A, Dietz AB, Womack JE, Stranzinger G, Fries R
(1993). Isolation and mapping of polymorphic microsatellites
in cattle. Anim Genet 24: 121124.
Vaiman D, Mercier D, Moazami-Goudarzi K, Eggen A,
Ciampolini R, Lepingle A et al (1994). A set of 99 cattle microsatellites: characterization, synteny mapping, and polymorphism. Mamm Genome 5: 288297.
Walsh PS, Metzger DA, Higuchi R (1991). Chelex 100 as a medium for simple extraction of DNA for PCR-based typing
from forensic material. Biotechniques10: 506513.
Weir BS, Cockerham CC (1984). Estimating F statistics for the
analysis of population structure. Evolution 38: 13581370. Whitlock R (1980). Rare Breeds the Vulnerable Survivors. Prism
Press: Chalmington, Dorchester.
Wright S (1951). The genetical structure of populations. Ann
Eugenics 15: 323354.
![]() Supplementary Information accompanies the paper on Heredity website ( http://www.nature.com/hdy).
Supplementary Information accompanies the paper on Heredity website ( http://www.nature.com/hdy).
