The official version of this document can be found via the PDF button.
The below content has been automatically generated from the original PDF and some formatting may have been lost, therefore it should not be relied upon to extract citations or propose amendments.
Report compiled by
Lara Luke BSc (Hons)Env Studs (Open)Dip Poll Con (Open) For Scrutiny Hearing, 23rd April 2009.
Introduction:
In The Strategic Plan 2005-2010, under Strategic Aim Four, the States address the issue of contaminated land with the pledge to ensure that unpolluted air, clean water and uncontaminated land exist as a basic right for all'.
The Island Plan 2002 recognises that fishing for shellfish, wet fish and fish farming are important economic activities, which need safeguarding and supporting.
Good water quality in areas where shellfish live is essential to prevent contamination of shellfish. Bottom feeders, such as oysters and mussels, can be particularly sensitive to pollution and contaminates as they are filter-feeders (drawing water across their sieve- like gills which can rapidly accumulate micro-organisms, heavy metals and organic contaminants).
The monitoring of the quality of shellfish waters helps to prevent them becoming contaminated, as there is a risk poor water quality could mean that shellfish become contaminated, which could then affect the people who eat them.
The situation we are faced with:
The excavation at the site of the incinerator is through material dumped at La Collette in its early phase when fly ash and bottom ash were mixed in with builder's rubble and other generally dumped items. The ash will have contained residue from electrical items and batteries etc with a high mercury content. (See section P 25 –Where is the ash from the incinerator?')
I trust that TTS would have the analysis of the soil which would have been prepared by the developers when they put in for the planning application for the construction of the energy from waste plant as the proposed development is built on landfill/ reclaimed land which is considered to be contaminated. However was a fresh soil analysis completed after the addition of the contaminated land from Castle Quays?
After monitoring the progress of the construction process it has been noted that these excavated pits both fill with sea water and empty in accordance with the tides. As the tide comes in it is filtering through the porous outer walls and in reverse through to low tide.
Mr. Tony Legg, oyster farmer and marine biologist, adds this information on hydropneumatic erosion:
"Hydropneumatic Erosion: This process warrants most consideration. It is the process that leads
to Blow Hole' formation on exposed cliff faces. Where wave action is severe, voids are formed within the eroded face. Subsequent wave action then compresses air within the void which can then expand explosively. Of interest is that this process can continue many tens of metres from the point of wave action and many meters above the high tide mark. (St. Catherine's Breakwater had a void 26 metres deep caused by this process, 25 km fetch). It may be that no area within the new reclamation is risk free from this process, including high level profiles."
What is the contaminated land likely to contain?
In the early 1990s, Jersey's Public Services Department, in response to concerns, commissioned Warr en Spring Laboratory to undertake an analysis of emissions from the incinerator. Included was a detailed analysis of the ash produced by the incinerator. The table I, reproduced below is to be found on page 29 of the WSL report –
Table 1
TABLE 9 –CONCENTRATIONS OF METALS IN COMBINED RESIDUAL ASH
METAL | UNIT 2 | UNIT 3 |
| µg g | µg g |
Mercury | 0.46 | 0.29 |
Vanadium | 62.3 | 56.1 |
Chromium | 212 | 244 |
Manganese | 937 | 937 |
Cobalt | 21.2 | 17.7 |
Nickel | 60.3 | 74.9 |
Copper | 18110 | 1160 |
Zinc | 6200 | 4540 |
Arsenic | 54.2 | 34.4 |
Selenium | 1.41 | 0.53 |
Cadmium | 33.5 | 34.0 |
Tin | 274 | 272 |
Antimony | 177 | 83.8 |
Thallium | 0.45 | 0.33 |
Lead | 4170 | 2020 |
Amongst the other information in the report are two tables – 11A and 11B – to be found on pages 30 and 31 of the WSL report. These two tables describe the CONCENTRATIONS OF DIOXINS AND DIBENZOFURANS IN COMBINED RESIDUE ASH", for units 2 and 3 respectively. These showed the total dioxin and dibenzofuran content of the combined ash from unit 2 to be 6.4112 ng-g, and a concentration of 0.5014 ng-g for unit 3.
Thus the WSL report demonstrates scientifically that the combined ash from the incinerator contains a variety of toxic heavy metals and polychlorinated compounds such as dioxins and furans which are a proven threat to human health. To further demonstrate this fact I reproduce below a section of a table titled "Toxic Effects of Common Hazardous Compounds". This is from page 476 of "Hazardous Wastes: Sources, Pathways, Receptors" by Richard J. Watts, published by John Wiley & Sons, ISBN 0-471-00238-0.
Table 2
Chemical | Acute Effects | Chronic Effects |
Polychlorinated Biphenyls PCB's | Minimal acute toxicity (0.5 g/Kg to 11.3Kg | Chloracne; increased liver enzymes; possible reproductive effects; act as cancer promoters |
Dioxins and Furans PCDDs/PCDFs | Chloracne, headaches, peripheral neuropathy | Induction of microsomal enzymes; altered liver metabolism; altered T-cell sub- sets; immunotoxicity; strongly implicated in carcinogenicity (may be a promoter) |
Inorganic Compounds Arsenic | Loss of blood, intestinal injuries, acute respiratory failure | Myelogeneous leukaemia, cancer of skin, lungs, lymph glands, bladder, kidney, prostate and liver |
Cadmium | Vomiting, cramping, weakness, and diarrhoea | Oral ingestion results in renal necrosis and dysfunction; induces lung, prostate, kidney, and stomach cancer in animals; no documented human cancer |
Hexavalent chromium | Readily absorbed by the skin where it acts as an irritant and immune-system sensitizer; oral absorption results in acute renal failure | Lung cancer |
Mercury | Central nervous system impairment including injury to motor neurons; renal disjunction | Central nervous system dysfunction, memory deficits, decrease in psychomotor skills, tremors |
Nickel | Not highly toxic, headache, shortness of breath | Immune system effects resulting in allergic contact dermatitis |
It appears that the WSL study did not test for polychlorinated biphenyls (PCBs) but it is likely that PCB is also present in the ash given its past use in electrical equipment. Only 5 of the 15 metal components of the ash identified in the WSL report are described in Table 2 so one must consider the fact of the existence of additional hazardous components in the ash to those listed in the table. (Source – proposition to the States by Senator Stuart Syvret, 10th June 2008)
Also important to note is that this analysis was compiled a number of years ago and since then, almost 20 years ago there has been a change in the type of waste that is incinerated. Think of the advancements in technology computers, mobile phones, i-pods, all of which are quite often updated and disposed of adding to the quantity of electrical waste. The same applies for televisions etc, plasma, LCD, all with their own disposal pollution aspects.
Definition of leachate:
This is the seepage of liquid through a waste disposal site, water passing through the ash/ contaminated land.
Leaching:
Draft Planning Advice Note
Development of Potentially Contaminated Land - Guidance for Developers, Agents and Consultants
Extract from above guidance:
What chemical tests should I carry out?
Chemicals tested for should include all contaminants of concern. Contaminants suspected to be present on the site which are not part of a generic test suite (such as the ICRCL suite) should not be missed out of the analysis for this reason. You should also carry out leachability tests to assess how mobile a contaminant is and whether it may pose a risk to water resources. Laboratories used should preferably be UKAS (United Kingdom Accreditation Service) accredited for all tests on contaminants of concern which they carry out.
http://www.gov.je/PlanningEnvironment/Environment/Environmental+Protection/Conta minated+land/Development+of+Potentially+Contaminated+Land.htm
Leaching means the removal of water of any soluble constituent from the soil/ ash or from waste tipped on land, therefore it is the gradual dissolution of a material from a solid containing it, e.g. metal from ore, heavy metal ions from ash etc.
How do we know heavy metals are leaching into available water on site?
We know this is happening as a result of two sets of water samples collected by the Environment Department. One for the application of a discharge permit, Application Number DP(B)2009/03/01, and the samples from the accident where one of the liners on the incinerator site was accidentally damaged by a site worker.
Below I reproduce my representation for the application of the discharge permit which outlines a number of concerns:
20th March 2009
Re: Application Number DP(B)2009/03/01
The points I make below are my representation to be taken into account with reference to the application for a discharge notice made by SBC Limited.
- The salinity of the water.
Typical seawater has a salinity of 35 000 ppm of which 30 000 ppm is common salt (NaCl). Brackish water has a salinity range from approximately 1 000 ppm to 10 000 ppm.
Here we have a situation that has caused both seawater and rainwater to collect and mix together forming brackish' water.
In the application for the discharge permit there is no reference to the salinity of the water, which can be extremely detrimental to marine life as stated below:
Marine and river environments have obvious differences in water quality, namely salinity. Each species of aquatic plant and animal is adapted to survive in either marine, brackish, or freshwater environments. There are species that can tolerate both, but these species usually thrive best in a specific water environment. The discharge of brackish water into the surrounding waters, if done in large quantities and with any regularity, may alter the aquatic environment significantly. Fluctuations in salinity will result in changes in the community of animals and plants living in that location. It can cause either multiplication or disappearance of species causing an imbalance on ecosystems.
The salinity of the water must be established prior to discharging into the sea.
- Retrospective permit:
How can you issue a discharge permit retrospectively, from the end of February 2009? With the high levels of toxic substances present in the tested water from the pit (as detailed in the application) that must have already been discharged, does that not mean that the sea has knowingly been polluted which is an offence under the Water Pollution (Jersey) Law, 2000?
- Discharge rate:
With the two settlement tanks having a total capacity of 75 m3 and the maximum quantity that could be discharged in one day of 432 m3, that means that these tanks would have around 6 full water changes a day. How will these changes allow the silt to settle out? (Suspended solids in sewage would take around 2 hours to settle when stationary; however this will be a constant flow)
- Oils.
How will oils be intercepted, as there is no explanation in the application?
- Conductivity and hydrocarbon:
As the results of the analysis are not included with the application, when will they be available for the public to view, before or after the decision?
Where are the details for the hydrocarbon separator?
- Application
Application is not filled out correctly, Part A not completed (it says all applicants); I could see no discharge points or sampling points clearly marked on the map; I can see no distance and depth relative to low water mark as the discharge is to coastal waters.
- Temperature:
The maximum of the trade effluent to be discharged is 18C, however where are the average sea temperatures, and what are the likely effects from the difference in temperatures on coastal waters of the Ramsar site?
- Heavy Metals:
The presence of heavy metals in the sample of pit water would be there as a result of the seepage of water through the ash pit, collecting at the bottom, which is known as leachate.
The lack of suitable inland disposal sites has meant that Jersey has increasingly turned to reclamation as the sole means of disposing of solid waste, with incineration playing an important role in reducing the volume of waste and prolonging the life of reclamation sites. However the possibility of leachate entering the marine environment is very real. If this occurred locally it would constitute a breach of the Dumping at Sea Law Sea Fisheries (Miscellaneous Provisions) 1974. This has led to the ash being dumped since 1987 above mean high water level at the reclamation sites.
When such leaching is excessive and/or prolonged, the metals can become concentrated up a marine food chain. Some bivalves, for example, concentrate certain metals tens of thousand times above the ambient level (Brooks and Rumsby, 1965). In extreme cases, potential public health risks arise because of the ingestion of contaminated seafood.
In Jersey the common limpet and brown fucoid seaweed have been sampled since 1994 at five locations (ie West of Albert, La Collette, St Aubin, Corbiere and Gorey). Samples have also been taken from Havre des Pas and Les Ecrehous.'
Source: http://www.gov.je/Health/public_health/health_protection/pollution/Heavy+Metals.htm
Reading the above quote from the States of Jersey website, makes me question whether the application for a discharge permit is in fact legal as it states if this occurred locally it would constitute a breach of the Dumping at Sea Law Sea Fisheries (Miscellaneous Provisions) 1974'.
Why has mercury not been tested for?
As heavy metals bioaccumulate mercury would potentially have the highest adverse effect. Bacteria can change mecury in water to methylmercury which binds to the proteins in fish. The levels of mercury will be higher, the higher up the food chain you go. The main pathway of mercury to humans is through the food chain- fish. In all living things heavy metals accumulate and are stored quicker than they are broken down.
Out of the heavy metals tested for, lead, cadmium, arsenic and nickel are categorized as toxic substances. It is noted that antimony also has not been tested for.
Another omission in the category for substances undesirable in high amounts is nitrate, and phenols (highly toxic to living organisms, also can poison sewage treatment plants and taint water in very small concentrations).
Monitoring this discharge for heavy metals should not be allowed to cease if this discharge permit is granted.
The EEC has 13 Directives (1990) on water quality. Would this discharge comply with the Directives set down for shellfish in designated waters which require protection to make them suitable for fish life or to ensure that the quality of shellfish is suitable for human consumption?
Samples from pit:
Lead is 38.4 µg/l above Maximum Admissible Concentrations (MAC). Iron 13,400 µg/l above MAC.
Manganese is 270 µg/l above MAC.
Are these levels safe to be entering our coastal bathing waters?
Testing for pH levels and treating will not affect the levels of heavy metals present. How will the heavy metals be removed from the tanks?
- Where are the baseline studies from the point of discharge? Also the water that is to be
discharged to sea will need to have the same complete analysis as that done on the baseline seawater sample. This is to be able to make a meaningful comparison between the treated and untreated water. Baseline samples of the untreated seawater need to be taken as well at regular intervals.
- In the 2007 Bathing Waters report (Jersey) it states that the quality of water is falling around this area by the number of coliform bacteria present, would the added problem of this discharge not cause the quality to further deteriorate? The report suggested that further testing for E-Coli should be commissioned as a result of the coliform count.
- How will this discharge affect the large part of the Islands shellfish which is stored in viviers (seawater tanks) on the Victoria Pier, also the adjacent area to the west of La Collette (Elizabeth Castle) shellfish is also stored in nourrices (floating viviers) considering the high levels of heavy metals contained in the pit water?
- Why is only the pH being continuously monitored?
Analysis of Waste Water
To determine the appropriate effluent treatment required and to monitor performance it is essential to test surface and process water prior to any discharge from source:
- After collection
- During treatment
- Prior to final discharge
Whilst some tests might be site specific, depending on the industry, it is essential to analyse for:
- Suspended lead
- Dissolved lead
- The acidity/alkalinity, i.e. the ph value
- Heavy metals such as antinomy, copper, zinc, arsenic, cadmium and mercury
- Test for oil, grease and dissolved salts, particularly if the process requires the
use of caustic soda or other reagents that produce soluble heavy metal salts.
- Will dioxins be tested for in both discharge and sea water?
Mussels have an important role in the circulation of dioxins. Mussels increase the net deposition of these substances on the sea bottom and make them more available for organisms living at the bottom. In addition mussels increase the residence time of the substances in the water masses and accumulate the substances and excreted them again.
- Have organizations such as Ramsar, Save Our Shoreline and also the local fishing industry been consulted with regards to this application?
After reading, and giving careful consideration to the information contained within it, it is my opinion that this application has not given due consideration to the potential and highly likely adverse effects on the marine environment. There are not enough controls and remedies to prevent pollution of the sea from toxic substances.
Below is a copy of the lab report which was attached to the application:
With reference to the other issue of the accident and the water in-flow and trench, I have reproduced the correspondence about the incident:
25 March 2009 01:17 Dear William,
I would like to know who the 'regulator' is that took the samples for testing from the
initial excavation 'accident' that this application has arisen from. Where are these samples being analysed and on who's behalf? Also could you tell me the scope of the testing and when these test results will be available for public reading?
I would like to thank you in advance for your time and help.
Best Wishes, Lara Luke
Thu, 26 Mar 2009 17:59:19 +0000 Dear Lara,
Thank you for your email. To answer your question regarding the Regulator - that is me, and my team - Environmental Protection, under delegated powers from the Minister for Planning and Environment. We regulate a variety of environmental legislation, the most pertinent Laws in
this case being the Waste Management (Jersey) Law 2005 and the Water Pollution (Jersey) Law 2000.
We took 2 samples (one from the inflow and one from the ponded liquid) from liquids entering a trench at La Collette which had been excavated beside a hydraulically independent engineered cell containing ash from the incinerator.
I think it is important at this stage to avoid confusion, to advise you that the samples we took from this location are unrelated to the application made to discharge brackish waters from the site. The application has been made to facilitate the construction of the new EFW Facility and has no relevance to the trench samples.
 The samples were analysed at the States Analyst to aid our understanding of the likely contents and the results are as follows.
The samples were analysed at the States Analyst to aid our understanding of the likely contents and the results are as follows.
 Determinand 13/03/09/WP/001 13/03/09/WP/002 As 25 33 Cd 0.6 1.8 Cr 2 28 Cu 10 60 Hg results to follow
Determinand 13/03/09/WP/001 13/03/09/WP/002 As 25 33 Cd 0.6 1.8 Cr 2 28 Cu 10 60 Hg results to follow
Ni 60 80 Pb 12 97 Se results to follow
Zn 20 140 pH 8.41 10.32
Note: Analysis - Total metals in micrograms per litre.
I attach a report that we put together at the time for your interest and would advise that we continue to work with Transport and Technical services to ensure that proposed remedial works are to our satisfaction. Might I propose that we meet to discuss any issues you have in respect of both the trench excavation issue and the discharge consent application. I would be happy to hear from you to arrange this.
I hope this is of assistance to you, Yours sincerely,
William Peggie
Assistant Director - Environmental Protection
Report attached to above email:
POLLUTION REPORT 2009022 – LA COLLETTE SERVICE TRENCH EXCAVATION – 13 MARCH 2009.
Page 1 of 5
EXECUTIVE SUMMARY:
On Friday 13th March at 17.00, Environmental Protection (EP) were called by Transport and Technical Services (TTS) staff who advised them that there had been an escape of liquid from the direction of an Ash cell on site at La Collette into a newly excavated service trench. They were concerned about the possibility of the leak worsening and any potential impact the situation may have on surrounding controlled waters, specifically the Ramsar site area to the east of the reclamation site.
On attendance at the site EP determined that there was no visible pollution to the marine environment which was some distance away and that seepage from the direction of the ash cell was contained either in the trench or in the surrounding soils.
Responsibility now lies with TTS and their contractors to keep a watching brief on the situation and to provide a remediation plan which must be agreed with Environmental Protection prior to commencement of those works.
EP Officers attended in their capacity as regulators of the Waste Management (Jersey) Law 2005 and the Water Pollution (Jersey) Law 2000. During the course of the site visits, it became apparent that no pollution to controlled waters or breaches of the Waste Management (Jersey) Law 2005 had occurred hence the generation of this report as EP do not intend to pursue any formal action at this time. This situation may change in future if there is any change in the site circumstances.
BACKGROUND
On Friday 13th March a TTS sub contractor on EFW groundwork was reported to have excavated through the edge of a cell containment membrane whist digging a service trench along the northern edge of the La Collette site between the Jersey Electricity compound and an area of historic ash disposal.
It was further reported that a small quantity of potentially contaminated water had leaked into the trench. This leak had reportedly been ongoing for between two to five hours and there appeared to be, at the time of the site visit, in the region of six to seven bucketfuls of liquid in the bottom of the trench. See Photo 1.
The liquid was seeping from the cell-side wall of the trench in a central section of the excavation and the rate of flow was estimated by TTS at the time to be 6 litres per minute though this was not measured or substantiated. The water ponded in the trench and at one end for a matter of seconds appeared to drain beneath the base to the surrounding soil. To stem this flow the contractors used a shovel to create a dam with existing soil from the base of the trench and cement which was added to solidify the mix. See Photos 2 and 3:
Environmental Protection officers took 2 samples, reference nos: 13/03/09/WP/001 and 13/03/09/WP/002. These were delivered to the States Analyst on 16 March 2009 for analysis of pH and a suite of metals. Results are expected within a week.
The actions taken to stem the flow of liquid were firstly to shore the edge of the excavation with timber: See Photo 4. and then to apply suction to the cell via a riser pipe (integral to the construction of the cell) by vacuum tanker: See Photo 5. The application of suction immediately resulted in the cessation of the seepage of liquid to the trench. It was agreed in discussions on site between EP, TTS, and TTS Contractors that more liquid would be removed in this manner during the course of the evening to ensure that levels in the cell did not get to the point that would result in further seepage. EP Officers requested that the site Project Manager called to update on the situation the following day. A call was received at 09.50 from him to report that a further 4 loads of liquid had been removed during the course of Friday evening and that on Saturday morning there was no evidence of seepage to the trench.
POLLUTION REPORT 2009022 – LA COLLETTE SERVICE TRENCH EXCAVATION – 13 MARCH 2009.
Page 2 of 5
CURRENT SITUATION:
An officer attended site on the afternoon of 16 March and confirmed that the situation had not changed and that there was no water in the trench. Discussions have been held between EP and TTS and a remediation plan has been requested from TTS prior to any remedial works or further project works on this section of excavation taking place. Within this an explanation must be provided in respect of how and why the breach of the liner occurred and explaining how TTS propose to prevent a reoccurrence. The plan should also describe in detail how TTS propose to repair the liner and reengineer the cell to provide containment of potential pollutants in the ash cell. They should finally indicate when this will take place and what timescales are proposed. This report has been requested verbally and TTS have agreed to supply.
WP. 16/3/09
POLLUTION REPORT 2009022 – LA COLLETTE SERVICE TRENCH EXCAVATION – 13 MARCH 2009.
Page 3 of 5
Photo 1 Photo 2
POLLUTION REPORT 2009022 – LA COLLETTE SERVICE TRENCH EXCAVATION – 13 MARCH 2009.
Page 4 of 5
Photo 3 Photo 4
POLLUTION REPORT 2009022 – LA COLLETTE SERVICE TRENCH EXCAVATION – 13 MARCH 2009.
Page 5 of 5
Photo 5
Sun, 29 Mar 2009 03:28:44 +0100 Dear William,
I would like to thank you for your detailed reply.
After reading through all the information that you have given me I do have a couple more questions for you:
- Which sample was taken from where, this is not indicated. I assume that you and your team would have got to the incident as soon as you could, and would have taken the samples on arrival. The concerning aspect of this is that it is the same water, in-flow and trench, therefore one would assume similar results for heavy metals. However these samples highlight a remarkable difference. Why could this be? The only other place this 'same' water has been is in the trench, so either these metals were absorbed into the water extremely quickly or the soil is highly contaminated. I trust that TTS would have the analysis of the soil which would have been prepared by the developers when they put in for the planning application for the construction of the energy from waste plant as the proposed development is built on landfill which is considered to be contaminated. Do you have a report on the analysis of the soil on the site?
- Do you have the results for mercury and selenium levels yet?
- Am I able to have a copy of the remediation plan?
- Are tidal waters entering the site at any point? If the sea was entering the site, isn't it highly likely that controlled waters would be at a high risk of being polluted especially by heavy metals?
- Where has the water come from?
- Has the salinity been tested?
- Is it possible that other areas of the ash pit membrane's could be damaged causing
leakage?
I would like to thank you in advance for answering my questions. Yours Faithfully,
Lara Luke
I am currently waiting for a response to this email.
From the samples that I have been able to see, they indicate that there is a problem with the leaching of heavy metals into the available water, which therefore indicates that the same leaching effect will be happening when the tidal flow both enters and leaves the excavated site. This leaves the surrounding controlled waters as the receptor for pollution.
What are the pathways for pollutants to enter the sea?
There are two main pathways, point source and non-point source.
Non-point source:
There's a type of pollution that degrades bodies of water and it's called nonpoint source (NPS) pollution because it doesn't come from a single source, or point, such as a sewage treatment plant or an industrial discharge pipe.
NPS pollution occurs mainly through storm water runoff. When it rains, runoff from farmland, city streets, construction sites, and suburban lawns, roofs and driveways enters our waterways. This runoff often contains harmful substances such as toxics, excess nutrients and sediments. NPS pollutions effects seldom show up overnight -- they often go unnoticed for years. This characteristic makes it all the more difficult to control. There are four major forms of NPS pollution: sediments, nutrients, toxic substances and pathogens.
Sediments - are soil particles carried by rainwater into streams, lakes, rivers and bays. By volume, sediment is the greatest pollutant of all. It's caused mainly by erosion resulting from bare land, poor farming practices, and construction and development.
Nutrients – are substances which help plants and animals live and grow. NPS officials are most concerned about excessive amounts of two nutrients; nitrogen and phosphorus. Fertilizer and animal waste are the main sources of these substances.
Toxic substances - are chemicals which cause human and wildlife health problems. They include organic and inorganic chemicals and metals, pesticides, formaldehyde, household chemicals, gasoline, motor oil, battery acid, roadway salt and so on.
Pathogens - are disease-causing microorganisms present in human and animal waste.
Most pathogens are bacteria.
Point source:
This is direct discharge of a pollutant(s) into a body of water, which can be directly linked, to for example a pipe.
Applying this to the current situation we have the nonpoint pathway, through the outer walls of the reclamation site, and through the infill and back out in to the sea, and also the possibility of treated' water being discharged at point source.
Life in bays could not exist without nutrients, but too much of a good thing often causes more harm than good. Nutrients over-enrich our waterways causing algal blooms which deplete oxygen. This makes the oxygen unavailable to fish and shellfish so they suffocate and die. The algae also cloud the water and coat underwater vegetation, cutting much needed sunlight. Sediment clouds water too, and it obstructs waterways, clogs sewers, interferes with navigation, and smothers fish and shellfish spawning grounds. Natural erosion and sedimentation occur at a lower rate than that resulting from human land use activities.
Underwater plants and aquatic animals are particularly threatened by NPS pollution. Oysters, shad, herring, striped bass and submerged aquatic vegetation -- considered by many to be the foundation of a stable aquatic ecosystem -- are damaged by this pollution.
Was this potential hazard identified in the Environmental Impact Assessment for the proposed incinerator?
Yes, please read extract from the EIS:
16.3 Impacts and Potentially Polluting Processes During Construction
16.3.1 Source Pathway Receptor Analysis
The following considerations apply to the proposed Energy from Waste facility:
- no rivers or streams would be affected by the proposed facility;
- the existing ditches, drains and settlement lagoons would either be used for the settlement of surface runoff or replaced with similar facilities during the construction period;
- mechanisms to prevent runoff during construction including fuel tank bunding, settlement lagoons etc,
- no private water supplies, surface water or borehole abstractions would be affected; and
- the works would not increase the risk of flooding.
The potential sources of pollution to coastal waters would be both during construction and operation of the site. The potential pathway is infiltration into the fill and then dispersal due to tidal movements affecting intrusion of water under the fill area.
At the detailed design stage trial pits and boreholes would be included as part of the ground investigation in order to allow testing for contaminants and determine the exact nature and depth of the fill in the area of the proposed foundation works. It is proposed that a "watching brief" for contaminated or hazardous materials should be adopted during the site development and construction phases. Any visual and olfactory evidence of contaminated or hazardous materials will result in further investigations being undertaken which may include laboratory testing and the material suitably dealt with by measures which may include containment or in exceptional circumstances disposal of the Island. The main receptor is the coastal waters which are designated as the South East Coast of Jersey RAMSAR Site, due to its high ecological value and diversity of habitats. This RAMSAR site would potentially be vulnerable should pollutants be released during construction or operation of the site. Groundwater beneath the side is also vulnerable but it is assumed for this assessment that it is in hydraulic continuity with the sea.
The design of the facility would aim to break the links between sources of pollution during construction and operation and the receptor which is the coastal RAMSAR site.
The potential hazards have been identified but no risk assessment has been properly conducted. There is no application of the precautionary principle, in fact other than stating what could happen, and has, no remediation plan has been considered or discussed in the environmental impact statement.
This is a breach of the Planning and Building (Jersey) Law 2002; please see Appendix 1 for the breakdown of the law.
It concerns me that in the EIA, it mentions that the existing drains may be used for surface run off etc. Given the level of contamination in the area, this could potentially also pollute our sewerage system. In some older landfills, leachate was directed to the sewers but this can cause a number of problems. Toxic metals from the leachate passing through the sewage treatment plant concentrate in the sewage sludge making it difficult or dangerous to dispose of to land without incurring a risk to the environment.
As sewage treatment works discharges are being improved throughout Europe and many other countries, the sewage treatment works operators are finding that leachates are difficult waste streams to treat because they contain very high ammoniacal nitrogen concentrations, they are usually very acidic , they are often anoxic and, if received in large volumes relative to the incoming sewage flow, the lack of Phosphorus in particular can result in nutrient starvation for the biological communities that perform the sewage treatment processes making leachate a difficult to treat waste stream. However, within aging municipal solid waste landfills this may not be a problem as the pH returns close to neutral after the initial stage of acidogenic leachate decomposition. Also many older leachate streams also contained a variety of synthetic organic species and their decomposition products, some of which had the potential to be acutely damaging to the environment.
Where is the ash from the incinerator?
In many respects this is a difficult question to answer. Before 1995, the incinerator ash (bottom and fly ash) was simply dumped along with inert waste in the West of Albert site, therefore it could be anywhere and in fact parts have been excavated and have now been transported to the La Collette Phase II site to various locations. Since 1995 the ash
was required to be placed in lined pits. However in the early days no records were kept of the position of the pits. (See Appendix 2 – Answers to Senator Stuart Syvret's proposition, question vii).
The excavation at the site of the incinerator is through material dumped at La Collette in its early phase when fly ash and bottom ash were mixed in with builder's rubble and other generally dumped items. The ash will have contained residue from electrical items and batteries etc with a high mercury content.
Even though since 1995 separate ash pits were created they actually ''sit' upon material as previously described and the sea is constantly washing below this material. Now of course the sea action is made worse by intrusion and opening the voids/fissures etc. The sea is now directly available to all levels of the fill and not just the bottom. The leaching rate will have increased as a result. Also these pits are lined with strengthened polythene sheeting which is overlapped and held in place with tyres. This type of construction would not prevent the leaching of various pollutants down through these pits from rain water etc and also would not prevent tidal surges from entering them from below. This leachate would be then flushed into the surrounding controlled waters. This does not take in to account any damage that may have occurred to the liners, whereby it would exacerbate the problem.
Former States Medical Officer of Health, Dr. John Harvey, and Health Protection Officer, Steve Smith, co-authored a report titled: Health Impact of the West of Albert Pier Reclamation Site.' Below is a quote from the report compiled in 2001 –
"Recent published studies have recognised the possible cumulative and synergistic effect of multiple hazardous agents, and have looked at the effect of exposure to hazardous sites, not individual toxins. The exposure risk is residence near to contaminated sites.
"The risk of adverse birth outcomes has been the focus of two such studies. A study of all residence near landfill sites in Great Britain showed small excess risks (c.10- 20%) of some congenital anomalies and low birth weight. This was not greater near sites with special waste (i.e. known toxic waste such as incinerator ash) possibly because these sites were subject to strict regulation. The authors noted that the small excess could be due to residual confounding (unmeasured effect of deprivation) or data artefacts. Another Europe wide study showed higher levels of risk for congenital anomalies. This study, known as EUROHAZCON used data from 7 registers in 5 countries. It showed an increased risk (2 – 3 times higher) for mothers living within 3km of landfill sites.
"A study in Canada showed increased risks of certain cancers for men living near solid waste landfill sites. The increases of twice the risk were shown for cancer of the pancreas in men living within 1.25km, cancer of the liver for those living within 1.5km, and non-Hodgkin's lymphoma within 2km."
Some 8 years ago health effects were being acknowledged by the States, why with today's knowledge and technology are there no proper controls on dealing with waste such as incinerator ash and its safe disposal?
Chemical pollution of the Aquatic Environment by Priority Pollutants
Any chemical in high enough concentrations can become a pollutant; however there are some chemicals that can be selected as being high priority for control in the aquatic environment because of their capability of exerting adverse effects even in low concentrations.
The red list' is a list of 23 dangerous substances, designated in the UK whose discharges to water should be minimised whilst using the BATNEEC (best available technology not entailing excessive costs) principle.
Red list substances | Additional substances on priority list |
Mercury and its compounds Cadium and its compounds Gamma-hexachlorocyclohexane DDT Pentachlorophenol Hexachlorobenzene Hexachlorobutadiene Aldrin Dieldrin Endrin Polychlorinated biphenyls Dichlorvos 1,2-Dichlorethane Trichlorobenzene Atrazine Simazine Tributyltin compounds Triphenyltin compounds Trifluralin Fenitrothion Azinphos-methyl Malathion Endosulphan | Copper Zinc Lead Arsenic Chromium Nickel Chloroform Carbon tetrachloride Azinphos-ethyl Fenthion Parathion Parathion-methyl Trichloroethylene Tetrachloroethylene Trichloroethane Dioxins |
![]() If extra background information on UK legislation for chemicals, please visit the website below: http://www.ukooaenvironmentallegislation.co.uk/Contents/topic_files/offshore/Productio n_chemicals.htm
If extra background information on UK legislation for chemicals, please visit the website below: http://www.ukooaenvironmentallegislation.co.uk/Contents/topic_files/offshore/Productio n_chemicals.htm
SEPA discharge consents
SEPA is responsible for the regulation of discharges to controlled waters under Part II of the Control of Pollution Act 1974 (as amended) and for the granting of consents and service of instruments to discharge under that Act. It inherited these functions under the Environment Act 1995 from the river purification authorities.
Since its inception, SEPA has been formulating policies to discharge these responsibilities, including :
Consenting policy for discharges to controlled waters (SEPA Policy No. 3, Version 1, July 1996) (draft);
Microbiological standards in marine waters (excluding shellfish waters) in relation to design criteria for discharges (SEPA Policy No. 27, Version 1, September 1998);
Initial dilution and mixing zones for discharges from coastal and estuarine outfalls (SEPA Policy No. 28, Version 1, September 1998).
Point source discharges for which consents are required include sewage and industrial discharges from non-prescribed processes (trade effluent). Prescribed processes require an IPC authorisation which is also issued by SEPA. Discharges from marine fish farm installations are considered to be trade effluent and require a discharge consent.
The procedure for the application for a discharge consent and the form of consent conditions (95 percentiles, upper tiers and absolute limits) is similar to that in England and Wales.
There is a requirement for all existing discharges to meet the following statutory requirements:
EC Bathing Waters Directive and UK Regulations (appropriate mandatory standards must be met at designated bathing waters);
EC Urban Waste Water Treatment Directive and UK Regulations (the appropriate level of treatment should be applied to the sewage, based on the population equivalent of the agglomeration served by the sewerage system);
EC Dangerous Substances Directive and UK Regulations (EQSs for List I and II substances in receiving waters must be met, including standstill provisions);
EC Shellfish Directive and UK Regulations (appropriate standards must be met for designated shellfish waters).
North Sea Conference and OSPAR commitments (reduction in loads of toxic substances in discharges to the marine environment must be demonstrated).
SEPA=s policy on initial dilution and mixing zones (SEPA Policy No 28) sets out requirements to be met in the design of new or modified discharges.
For discharges with greater than 100 population equivalent, outfalls should be designed and constructed to provide the following minimum initial dilution to reduce both the visibility of density slicks and the occurrence of smell nuisance to acceptable levels:
minimum initial dilution of 100 times (95 percentile) for primary treated effluent;
minimum initial dilution of 50 times (95 percentile) for secondary treated effluent, including septic effluent;
minimum initial dilution of 50 times (95 percentile) for significant new or modified industrial discharges (to be judged on an individual basis).
Modelling studies are required to determine the best location and design of the outfall and to demonstrate achievement of the minimum initial dilution requirements. SEPA specifies the requirements for modelling studies and recommends the use of one of 3 models: ELSID, PLUMES or CORMIX (see SEPA Policy No. 28).
Requirements for mixing zones are also specified to inform the design of an outfall and the consent conditions to be set to achieve compliance with statutory requirements and to protect the environment. These requirements include:
a limitation on the size of a mixing zone to 100 m in any direction;
UK or SEPA Environmental Quality Standards (EQSs) should not be breached
outwith the mixing zone;
where toxicity-based criteria are used, there should be no residual toxicity outwith the mixing zone;
neighbouring mixing zones should not merge and ideally should be at least 100
m apart;
no mixing zone should impinge on the Mean Low Water Springs (MLWS) shoreline;
no mixing zone should plug an estuary, sea loch or small bay;
the mixing zone will not be allowed to jeopardise the integrity of any European marine site;
the mixing zone should not give rise to significant slicks or other aesthetic problems;
accumulation of solids on the sea bed must not threaten the achievement of standstill clauses for List I substances outwith the mixing zone and not cause acute toxic effects to sediment-dwelling organisms within the mixing zone.
SEPA has developed a policy for microbiological standards in marine waters (except shellfish waters) in relation to design criteria for discharges (SEPA policy No 27). This policy has some influence on all marine waters in Scotland.
For all marine waters, no new or modified discharges will be allowed to result in deterioration of the class which is currently achieved under the coastal classification scheme nor threaten progress in improving class C and D marine waters identified in SEPA's corporate plans.
For identified bathing waters, existing discharges must enable mandatory standards to be achieved and, for new or modified discharges, the outfalls must be designed so as to achieve guideline standards.
SEPA may also designate >recreational waters= where significant water contact activities are practised outwith identified bathing waters. SEPA will require mandatory microbiological standards to be achieved at relevant times of year and promote the achievement of guideline standards where appropriate.
SEPA will also adopt a strong presumption that mandatory microbiological standards are achieved at 'shoreline waters' (i.e. those visited by the public).
As in England and Wales, the primary driver for the derivation of consent conditions is compliance with statutory requirements, in particular bathing water, Urban Waste Water Treatment and shellfish water standards. SEPA has supplemented these requirements in Scotland to some extent with their policies. These policies enable Scott ish Natural Heritage (SNH) to verify that consent conditions for new or modified discharges in or close to European marine sites have been set according to the procedures outlined in
these documents.
The approach to the regulation and monitoring of cage fish farming in Scotland has been comprehensively laid out in a manual of procedures (SEPA 1998). This manual provides details of the application process, the assessment of the application, setting consent limits, process for granting and refusing a consent, monitoring, data management and use of information and review of consents. Conservation agency staff should have access to this document to ensure that all cage fish farming consents have been set according to the process.
Source: http://www.ukmarinesac.org.uk/activities/water-quality/wq2_2_1.htm
General Information on shellfish:
The shellfish family is composed of gastropods, with a single-piece shell (sea snails, whelks, barnacles, abalone, etc.) and bivalves, with a shell made of 2 distinct parts (oysters, mussels, clams, scallops, etc.).
Shellfish have an especially high mineral element content (calcium, magnesium) and oligo-elements (zinc, iron, iodine, selenium). They are also high in certain vitamins, like vitamin B, vitamin D and vitamin E. Shellfish are low in calories (60 to 80 KCAL/100g of meat), high in protein (10 to 15 g/100 g) and low in fat (1 to 2 g/100 g). Like fish, they contain lipids made up primarily of fatty acids that are good for the cardiovascular system. Their cholesterol content is on par with that of meats (50 to 80 mg/100g).
Shellfish can concentrate up to 100 times the bacterial and virus content present in seawater, thereby explaining why it can be harmful to your health to eat shellfish from polluted waters. They also contain high concentrations of toxic plankton, chemical
compounds such as heavy metals (lead, mercury, cadmium) and certain organic compounds (hydrocarbons, polychlorobiphenyls – PCBs, tributyl tin – TBT, certain pesticides, etc.).
Long-term classification of shellfish harvesting areas: Classification of areas (Class A, B, C or prohibited)
As with previous hygiene legislation, under the new Hygiene Regulations European Union Member States are still required to put in place a programme for monitoring and classifying shellfish harvesting areas. Production areas are categorised by the level of microbiological contamination, namely the level of E. coli contamination found in shellfish sampled from a site. These areas are classified as Class A, B, C or prohibited: Class A - shellfish contain less than 230 E. coli per 100 grams of flesh
Class B - shellfish contain less than 4,600 E. coli per 100 grams of flesh
Class C - shellfish contain less than 46,000 E. coli per 100 grams of flesh
Prohibited area - above 46,000 E. coli per 100 grams of flesh
(Information from the Food Standards Agency)
It appears from the recent press release of Dr. Rosemary Geller, that she has omitted a class – Prohibited area.
States working together with fishermen to ensure safety of local shellfish
Press Release 1st April 2009
Over the last years there has been a change in the classification of some of shellfish harvesting areas in Jersey . This means that products need to be relayed or purified before they can be marketed for human consumption.
The actual cause of this remains unknown but is consistent with the trends in the UK and other European countries. It has been noted that there has been a change in weather patterns resulting in unusually heavy rainfall and this may be correlated with impacts on beach areas and the shellfish farms.
States officials for Health, Environment and Transport and Technical Services have been working together with the industry to try to identify any possible issues that could be impacting on local shellfish farms and a large scale investigation continues to be carried out.
The local shellfish farmers routinely provide samples for classification purposes and for the research work that is being undertaken.
The classification of the areas is based on food safety standards for E coli and all business operations are approved under European food regulations.
Dr Rosemary Geller , Medical Officer of Health said:
"We are committed to supporting the shellfish industry in identifying any possible impacts on harvesting areas. A range of sampling techniques has been employed but this is a complex issue and it will take some time to complete the research.
My officers are working closely with the businesses affected and are putting appropriate public health measures in place so that the public can be confident that any products marketed for human consumption meet food safety standards."
Food law across Europe, defines raw oysters as a high risk food', and as such they require strict food hygiene controls. The UK Food Standards Agency recommends that raw oysters can be cooked to improve their safety.
Notes
The classification of harvesting areas is based on a food safety standard for E coli and is determined on an annual basis following a minimum of 12 monthly samples. Class A – less than 230 E coli/100g flesh – product can be eaten raw
Class B – 231-4,600 E coli/100g flesh – the product must be purified, relayed or cooked to meet class A standards
Class C – over 4,600 - product must be relayed purified, cooked to meet Class A standard.
High risk foods' are those that support the growth of pathogenic microorganisms and are ready to eat.
Bathing water:
Other problems with sea water:
Assessment of
bathing water quality
for the States of Jersey 2007
A Report to
Environmental Protection, States of Jersey Dr Mark Wyer
Professor David Kay
February 2008
Final Report
1.3 Compliance assessment
Table 1.1 lists the raw data for bacterial concentrations at each location.
Concentrations were compared with the following Imperative or Guide values specified in Directive 76/160/EEC:
Standard
Organism Imperative Guide
Total coliform 10,000 cfu/100ml 500 cfu/100ml
Faecal coliform 2,000 cfu/100ml 100 cfu/100ml
Faecal streptococci -- 100 cfu/100ml
Any results exceeding these values are highlighted in Table 1.1, bold figures indicating Guideline exceedence and bold/underlined figures Imperative exceedence. The Directive specifies that 95% of samples (i.e. nineteen samples in twenty) must show bacterial counts below the Imperative criteria defined for total and faecal coliforms. The Guide Standards for the two coliform parameters require 80% of samples (i.e. sixteen samples in twenty) not to exceed the specified values. In the case 2 of faecal streptococci, the Guide criterion can be exceeded in 10% of samples (i.e. two in twenty samples) through the bathing season. Temporal plots of water quality at each location, along with corresponding daily rainfall, are shown in Figures 1.2 and 1.3. Any values above or below the respective upper and lower limits of detection have been plotted as the detection limit values in these plots. The proportions (%) of samples complying with the EC Directive criteria for bacterial parameters at each location are listed in Table 1.2.
All sixteen of the bathing water monitoring locations complied with the Imperative standards. A further summary of the compliance of Jersey bathing waters with EC standards is given in Figures 1.4 to 1.6, which provide maps showing compliance for each of the three faecal indicator organisms. Three results exceeded the Imperative standard concentration of 2,000 cfu/100 ml specified for faecal coliforms. These were from: St Ouen Le Braye (19/06/2007), Victoria Pool (09/07/2007) and La Haule (24/09/2007). The sample from St Ouen Le Braye also had a total coliform concentration in excess 10,000 cfu/100ml. Both concentrations in this sample exceed the upper limit of detection for the coliform parameters (20,000 cfu/100 ml). This result was extremely unusual for this location, which demonstrated low coliform concentrations in all other samples through the 2007 bathing season and has consistently demonstrated low concentrations during past bathing seasons. In addition, the sample from 19/06/2007 exhibited a low faecal streptococci concentration (7 cfu/100 ml) whilst low concentrations were recorded for all three parameters at the St Ouen Watersplash location on the same day (<100 total
coliforms/100 ml, 10 faecal coliforms/100 ml and 3 faecal streptococci/100 ml). Weather conditions were fine on the sampling day in question. An additional sample taken as soon as possible after receiving the result (i.e. on 21/06/2007) demonstrated low concentrations for the two coliform parameters, below the lower limits of detection. These results suggest that the high total and faecal coliform concentrations from 19/06/2007 may not have resulted from faecal contamination (i.e. a corresponding elevated faecal streptococci concentration would have been expected in the sample). This could have been assessed by further microbiological analysis, for example confirmation of Escherichia coli from the faecal coliform plates. Such confirmatory analysis would provide some clue as to whether the organisms have a faecal provenance. Non- compliance with the Guideline criterion for total coliforms (i.e. 80% samples _ 500 cfu/100 ml) was restricted to La Haule, in the west of St Aubin's Bay on the south coast (Figure 1.4). This location also showed non-compliance with the Guideline criteria for the other two parameters (Table 1.2). In addition to La Haule, Guideline compliance failures associated with faecal coliforms were found at four further locations: Victoria Pool in the east of St Aubin's Bay, Grouville on the east coast, Bonne Nuit on the north coast and Rozel in the north east (Table 1.2, Figure
1.5). These locations were also non-compliant with the Guide standard for faecal streptococci (Table 1.2, Figure 1.6). Guideline non-compliance with the faecal streptococci parameter was evident at three further locations: Havre des Pas and
Green Island on the south east coast and Plemont in the north west of Jersey. Overall compliance with the Guide standards for the 2007 season was thus 7/16 (43.8%), which is the lowest level recorded in Jersey in recent years (68.8% to 87.5% between 2003 and 2006). Comparable figures for England and Wales ranged from (80.4% to 85.2% between 2003 and 2006 – see: www.environment-agency.gov.uk) and the Guideline compliance rate for 2007 was 79.8%.
3 Table 1.2 indicates that non-compliance with the faecal streptococci Guide criterion was the driver of the low overall level compliance with the Guide standards, with four of the nine non-compliant locations showing non-compliance with the faecal streptococci standard alone. Closer inspection of individual results with faecal streptococci concentrations exceeding 100 cfu/100 ml show some unusual patterns, with faecal streptococci concentrations exceeding the corresponding coliform parameter concentrations. Examples include La Haule, Victoria Pool, Grouville , Archirondel, Bouley Bay and Rozel on 14/05/2007 (Table 1.1, Figures 1.2 and 1.3). This pattern is also evident in samples from various locations on 15/05/2007 and is clearly implicated in the Guide compliance failures at Plemont and Green Island. Whilst it is unusual for faecal streptococci concentrations to exceed those of the coliform parameters such patterns have been observed elsewhere (Wyer et al., 1999). As with the sample exhibiting unusually high total and faecal coliform concentrations from St Ouen Le Braye discussed above, these unusual results could be explored through further microbiological analysis, for example the confirmation of intestinal enterococci. This would provide a more definitive answer as to whether the organisms originate from faecal contamination and are not environmental species of bacteria (e.g. aerobic spore bearing bacilli and Aerococcus viridans) that can contribute to presumptive faecal streptococci counts.
It is apparent from the decline in the quality of bathing water and the changes in the classification of designated shellfish harvesting areas that there is an environmental problem. This problem needs remediation before it is too late for many shellfish populations, and in turn the livelihoods of fishermen and public health. There is a steady decline so we must look at what is potentially the most environmentally hazardous source, and this is the reclamation site.
Heavy metals:
Heavy metals are individual metals and metal compounds that can impact human health. These are all naturally occurring substances which are often present in the environment at low levels. In larger amounts, they can be dangerous. Generally, humans are exposed to these metals by ingestion (drinking or eating) or inhalation (breathing). Working in or living near an industrial site which utilizes these metals and their compounds increases ones risk of exposure, as does living near a site where these metals have been improperly disposed. Subsistence lifestyles can also impose higher risks of exposure and health impacts because of hunting and gathering activities, such as fishing.
The most common are mercury, lead, cadmium, zinc, chromium and plutonium. Ecosystems can be affected by their discharge, e.g. marine and freshwater diatoms can lose 50 per cent of their growth rate at concentrations as low as 1 part per thousand million of mercuric compounds.
An important note on the leaching of heavy metals is the variation of each metal in terms of solubility. For example, if you took five samples with identical soil and water quantities but tested these samples at different times (say 5 hour intervals), you may have substantially different results. Therefore the samples from the trench may not be a true representation of the total quantities of heavy metals that are available to the water stream.
There are numerous, complex effects of heavy metals and varying reactions, which for the purpose of this report may be too in depth. However I would like to highlight the fact that copper can be highly toxic to some shellfish in small concentrations. Also there is a link between the increased absorption of some heavy metals and certain strains of e-coli, this is because the heavy metals are also absorbed by the e-coli bacteria. For further information on this please read Appendix 3.
In a paper written in respect of the then proposed dumping of ash in the south of La Collette site, Mr. Tony Legg considered the potential for Cadmium to leach –
"From the above observations it is evident that an oxidising environment that is high in chlorine, that fluctuates in salinity, that occasionally is anoxic with free sulphide ions, that has a water table comprised of tidal and field water capacity interstitial solute, and has energy put into the system by tidal movement of that solute, is very well suited to making cadmium bioavailable. In addition, the ash material is already in an oxidised state, thereby speeding up the process.
"In situ, the ash is likely to be just above the MHWS mark and some 2 meters deep with c. 1 meter overburden. After normal rainfall or windblown spray this zone will be at field capacity with water filling all available pore spaces. When the tide rises or falls this water will be moved and exchanged, salinities will change, waters will drain and cadmium enriched leachate will form."
Information on heavy metals from the States of Jersey Website:
Accessed 03/04/09, States of Jersey website, information on heavy metals: http://www.gov.je/Health/public_health/health_protection/pollution/Heavy+Metals.htm
Heavy Metals
The sampling for Heavy Metals is now carried out by the Environment Department.
In many industrialised countries, including Jersey, incineration of refuse is considered a convenient method of reducing waste volume since it commonly achieves a reduction of about 90%. However, in the broader environmental context, the process is not without disadvantages particularly with respect to the associated gaseous emissions.

The solid residue consists primarily of bottom ash (80 - 90%) from the furnace bottom and fly ash from the dust collection system. (see picture above) Whilst in some instances incineration residues may be treated as toxic waste (eg Canada), it is not uncommon for them to be disposed of by landfilling. However, in recent years there has been an increased concern for the potential hazards posed by leachate from such sites (eg Hjelman et al, 1988). Leachate would contain heavy metals such as cadmium, mercury and lead which can be toxic at relatively low concentrations.
The lack of suitable inland disposal sites has meant that Jersey has increasingly turned to reclamation as the sole means of disposing of solid waste, with incineration playing an important role in reducing the volume of waste and prolonging the life of reclamation sites. However the possibility of leachate entering the marine environment is very real. If this occurred locally it would constitute a breach of the Dumping at Sea Law Sea Fisheries (Miscellaneous Provisions) 1974. This has led to the ash being dumped since 1987 above mean high water level at the reclamation sites.
When such leaching is excessive and/or prolonged, the metals can become concentrated up a marine food chain. Some bivalves, for example, concentrate certain metals tens of thousand times above the ambient level (Brooks and Rumsby, 1965). In extreme cases, potential public health risks arise because of the ingestion of contaminated seafood.
In Jersey the common limpet and brown fucoid seaweed have been sampled since 1994 at five locations (ie West of Albert, La Collette, St Aubin, Corbiere and Gorey). Samples have also been taken from Havre des Pas and Les Ecrehous.
Please contact the Environment Department on Tel: 441600 for more information.
Bioaccumulation
Biological concentration is the mechanism whereby filter feeders such as limpets, oysters and other shellfish concentrate heavy metals or other stable compounds present in dilute concentrations in sea or fresh water. Animals can accumulate metals as well by eating plants, fish, or drinking water with elevated metal concentrations. These metals are not excreted by the animals; rather, they accumulate mostly in the organs as well as the skin, hair, and bones. Fish accumulate metals from the water they live in as well as from organisms they eat. Bottom feeders are particularly susceptible to metals bioaccumulation as they can ingest sediments laced with metals. Minute traces of toxic compounds can be concentrated biologically and enter the food chain of human beings.
Potential effects on human health of some heavy metals: Mercury
Mercury is the only metal which is liquid at ordinary temperatures. It rarely occurs free in nature and is a heavy, silvery-white liquid metal.
Once consumed, mercury and the bivalent metals are engaged in a continuous fight against one another which results in the replacement of the "lighter" element by the "heavier" one, in terms of their atomic masses. Replacement reactions, also called "fight for the site," occur when heavy metals grab the biological spaces that should be filled by necessary organic minerals. Just as carbon monoxide replaces essential oxygen, other elements and compounds cause their toxic effect by replacing chemicals essential to bio- chemical functions. Mercury, found in amalgam fillings, paints, and some industrial processes, is not recognized in having any use in the body. Mercury is not taken up by plants, however, it may turn up in food as it can be spread within food chains by smaller organisms which are consumed by humans, and one example is through fish. Concentrations of Mercury in fish usually greatly exceed the concentrations in their environment. Beef products can also contain eminent quantities of mercury. Mercury is not commonly found in plant products, but it can enter our bodies through vegetables and other crops, when sprays containing mercury are applied in agriculture.
Adverse Health effects of Mercury:
Mercury salts will compete with zinc in its bio-chemical reactions hence preventing zinc performing its functions in the body. Therefore the leaching of mercury into the body from whatever source will cause zinc deficient symptoms to appear such as
fatigue, PMS, thyroid problem, loss of smell and taste, macular degeneration, prostate enlargement, rheumatoid arthritis, sterility, immune suppression, etc., even if there is plenty of zinc available. Studies show that mercury is eight times more concentrated in the foetus than in the rest of the body. Direct exposure to mercury can cause lung
irritation, skin rashes, nerve, brain and kidney damage, eye irritation, vomiting and diarrhoea. Mercury and its many effects on our bodies at elevated levels can be simplified into the following main effects:
• Disrupting the nervous pathways;
• Damage to brain function, can cause degradation of learning abilities, personality changes, tremors, vision changes, deafness, and muscle in-coordination and memory loss;
• DNA damage and chromosomal damage - chromosomal damage is known to cause mongolism;
• Allergic reactions, resulting in skin rashes, tiredness and headaches;
• Negative reproductive effects, such as sperm damage, birth defects and miscarriages.
Regulatory limits:
EPA – 2 parts per billion parts (ppb) in drinking water
FDA – 1 part of methylmercury in a million parts of seafood.
OSHA – 0.1 milligram of organic mercury per cubic meter of workplace air and 0.05 milligrams per cubic meter of metallic mercury vapor for 8-hour shifts and 40-hour work week.
Cadmium
Cadmium is a very toxic metal. All soils and rocks, including coal and mineral fertilizers, contain some cadmium. Cadmium has many uses, including batteries, pigments, metal coatings, and plastics. It is used extensively in electroplating.
Cadmium, in industry is a by-product from the extraction of zinc, lead and copper. Cadmium is found in pesticides and manures therefore are seen to enter the environment from terra-forming. People's uptake of cadmium takes place mainly through food. Fish, plants and animals bio-accumulate. Examples: liver, kidney, meat, mushrooms, shellfish, mussels, cocoa powder and dried seaweed. An exposure to significantly higher cadmium levels occur when people use tobacco. The cadmium in tobacco smoke enters the bloodstream via the respiratory system and distributed to the rest of the body. Cadmium can severely damage the lungs and may even cause death.
Once the cadmium reaches the liver where it is bonded to protein forming complexes, which are then transported to the kidneys where accumulation causes damage to the filtration process. This damage allows essential proteins and glycol nutrients to be excreted from the body causing even further kidney damage.
Health effects that can be caused by cadmium are:
- Damage to the central nervous system
Damage to the immune system
Psychological disorders and possibly DNA damage
- Reproductive failure and possibly even infertility;
- Cadmium and cadmium compounds are known human carcinogens. Smokers get exposed to significantly higher cadmium levels than non-smokers. Severe damage to the lungs may occur through breathing high levels of cadmium.
- Ingesting very high levels severely irritates the stomach, leading to vomiting and diarrhea.
- Long-term exposure to lower levels leads to a buildup in the kidneys and possible kidney disease, lung damage, and fragile bones.
Regulatory limits
EPA – 5 parts per billion (ppb) or 0.005 parts per million (ppm) of cadmium in drinking water
Food and Drug Administration (FDA) – concentration in bottled drinking water should not exceed 0.005 ppm (5 ppb).
OSHA – an average of 5 micrograms per cubic meter of workplace air for an 8-hour workday, 40-hour work week.
Lead
As a result of human activities, such as fossil fuel burning, mining, and manufacturing, lead and lead compounds can be found in all parts of our environment. This includes air, soil, and water. Lead is used in many different ways. It is used to produce batteries, ammunition, metal products like solder and pipes, and X-ray shielding devices. Lead is a highly toxic metal and, as a result of related health concerns (see below), its use in several products like gasoline, paints, and pipe solder, has been drastically reduced in recent years. Today, the most common sources of lead exposure are lead-based paint and possibly water pipes in older homes, contaminated soil, household dust, drinking water, lead crystal, lead in certain cosmetics and toys, and lead-glazed pottery. Lead can be present in drinking water as a result of dissolution from natural sources, or from household plumbing systems containing lead. These may include lead in pipes, or in solder used to seal joins. The amount of lead dissolved will depend on a number of factors including pH, water hardness, and the standing time of the water.
Lead is the most common of the heavy metals and is mined widely throughout the world. It is used in the production of lead acid batteries, solder, alloys, cable sheathing, paint pigments, rust inhibitors, ammunition, glazes and plastic stabilisers. The organo-lead compounds tetramethyl and tetraethyl lead are used extensively as anti-knock and lubrication compounds in gasoline.
Adverse Health effects of Lead
Lead fulfils no essential function in the human body and is a probable human carcinogen. Lead can affect every organ and system in the body. Long-term exposure of adults can result in decreased performance in some tests that measure functions of the nervous system; weakness in fingers, wrists, or ankles; small increases in blood pressure; and anemia. Exposure to high lead levels can severely damage the brain and kidneys and
ultimately cause death. In pregnant women, high levels of exposure to lead may cause miscarriage. High level exposure in men can damage the organs responsible for sperm production.
Also:
• Behavioural disruptions of children, such as aggression, impulsive behaviour and hyperactivity.
• Disruption of the biosynthesis of haemoglobin and anaemia;
• Declined fertility of men through sperm damage;
• Diminished learning abilities of children;
• Loss of I.Q;
• Kidney damage;
• Rise in blood pressure;
• Disruption of nervous systems;
Regulatory limits:
EPA – 15 parts per billion (ppb) in drinking water, 0.15 micrograms per cubic meter in air.
Copper
Copper is widely distributed in rocks and soils as carbonate and sulphide minerals. Copper is relatively resistant to corrosion and is used in domestic water supply pipes and fittings. It is also used in the electro-plating and chemical industries, and in many household goods. Copper sulphate is used extensively to control the growth of algae in water storages. Copper can be found in many kinds of food and in drinking water, because of that, we absorb eminent quantities of copper each day by eating, drinking and breathing. Organic copper is necessary as a trace element that is essential for human health.
Long-term exposure to copper in the industry level can cause irritation of the nose, mouth and eyes and it causes headaches, stomach-aches, dizziness, vomiting and diarrhoea. Intentionally high uptakes of copper may cause liver and kidney damage and even death.
Some adverse health effects of copper :
• Insomnia;
• Depression;
• Hypo-tension;
• Acne;
• Heart disease;
• Pre-menstrual tension;
• Postpartum depression;
• Paranoid and hallucinatory schizophrenias
• Childhood hyperactivity and autism.
• Above 50mg/Kg body weight can be lethal.
Zinc
Zinc is a very common substance and many foodstuffs contain certain concentrations of zinc. Drinking water also contains certain amounts of zinc, which may be higher when it is stored in metal tanks. Organic zinc is a trace element that is essential for human health. People deficient in zinc absorption can experience a loss of appetite, decreased sense of taste and smell, slow wound healing, skin sores and even birth defects.
Adverse Health effects of Zinc
Some adverse health effects of Zinc when over exposed:
• Stomach cramps;
• Skin irritations;
• Vomiting, nausea, anaemia;
• Arteriosclerosis;
• Respiratory disorders.
Arsenic
Aside from occurring naturally in the environment, arsenic can be released in larger quantities through volcanic activity, erosion of rocks, forest fires, and human activity. Arsenic is also found in paints, dyes, metals, drugs, soaps and semi-conductors. Animal feeding operations and certain fertilizers and pesticides can release high amounts of arsenic to the environment as can industry practices such as copper or lead smelting, mining, and coal burning.
Some health effects:
- Arsenic is odorless and tasteless. Inorganic arsenic is a known carcinogen and can cause cancer of the skin, lungs, liver and bladder.
- Lower level exposure can cause nausea and vomiting, decreased production of red and white blood cells, abnormal heart rhythm, damage to blood vessels, and a sensation of "pins and needles" in hands and feet.
- Ingestion of very high levels can possibly result in death.
- Long-term low level exposure can cause a darkening of the skin and the appearance of small "corns" or "warts" on the palms, soles, and torso.
Fish and shellfish can accumulate arsenic and is also toxic to plants at levels not harmful to humans and animals.
Regulatory limits:
Environmental Protection Agency (EPA) - 0.01 parts per million (ppm) in drinking water.
Occupational Safety and Health Administration (OSHA) - 10 micrograms per cubic meter of workplace air (10 µg/ m3) for 8 hour shifts and 40 hour work weeks.
Barium
Barium is a very abundant, naturally occurring metal and is used for a variety of industrial purposes. Barium compounds, such as barium-nickel alloys are used for spark- plug electrodes and in vacuum tubes as a drying and oxygen-removing agent; barium sulfide is used in fluorescent lamps; barium sulfate is used in diagnostic medicine; barium nitrate and chlorate give fireworks a green color. Barium compounds are also used in drilling muds, paint, bricks, ceramics, glass, and rubber. Barium accumulates in certain plants, seaweed, and fish.
Health effects
Barium is not known to cause cancer.
Short term exposure can cause vomiting, abdominal cramps, diarrohea, difficulties in breathing, increased or decreased blood pressure, numbness around the face, and muscle weakness.
Large amounts of barium intake can cause, high blood pressure, changes in heart rhythm or paralysis and possibly death.
Regulatory limits:
EPA - 2.0 parts per million (ppm) in drinking water.
OSHA - 0.5 milligrams of soluble barium compounds per cubic meter of workplace air for 8 hour shifts and 40 hour work week.
Chromium
Chromium is found in rocks, animals, plants, and soil and can be a liquid, solid, or gas. Chromium compounds bind to soil and are not likely to migrate to ground water but, they are very persistent in sediments in water. Chromium is used in metal alloys such as stainless steel; protective coatings on metal (electroplating); magnetic tapes; and pigments for paints, cement, paper, rubber, composition floor covering and other materials. Its soluble forms are used in wood preservatives. Chromium has a high potential for uptake by aquatic life, especially bottom-feeders.
Health effects:
Chromium (VI) compounds are toxins and known human carcinogens, whereas Chromium (III) is an essential nutrient. Breathing high levels can cause irritation to the lining of the nose; nose ulcers; runny nose; and breathing problems, such as asthma, cough, shortness of breath, or wheezing. Skin contact can cause skin ulcers. Allergic reactions consisting of severe redness and swelling of the skin have been noted. Long term exposure can cause damage to liver, kidney circulatory and nerve tissues, as well as skin irritation.
Regulatory limits
EPA– 0.1 ppm (parts per million) in drinking water.
FDA – should not exceed 1 milligram per liter (1ppm) in bottled water.
OSHA – an average of between 0.0005 and 1.0 milligram per cubic meter of workplace air for an 8-hour workday, 40-hour workweek, depending on the compound.
Selenium
Selenium is a trace mineral widely distributed in most rocks and soils. Processed selenium is used in the electronics industry; as a nutritional supplement; in the glass industry; in plastics, paints, enamels, inks, and rubber; in the preparation of pharmaceuticals; as a nutritional feed additive for poultry and livestock; in pesticide formulations; in rubber production; as an ingredient in antidandruff shampoos; and as a constituent of fungicides. Radioactive selenium is used in diagnostic medicine.
Selenium Biocaccumulates readily; Indicator plants for high selenium concentrations in soils are: milkvetch or locoweed (Astragalus), prince's plume (Stanleya), woody aster (Xylorhiza, sp.) and false goldenweed (Oonopsis, sp.) These plants require high selenium levels to thrive. High concentrations are found in kidney, tuna, crab and lobster.
Health effects:
Selenium is toxic in large amounts, but trace amounts of it are necessary for cellular function in most, if not all, animals. For humans, selenium is an essential trace nutrient. For example, selenium plays a role in the element functioning of the thyroid gland. The Tolerable Upper Intake Level is 400 micrograms of selenium per day. Consumption above that level can lead to selenosis. Short-term oral exposure to high concentrations can cause nausea, vomiting, and diarrhea. Chronic oral exposure to high concentrations can produce selenosis. Major signs of selenosis are hair loss, nail brittleness, and neurological abnormalities. Brief exposures to high levels in air can result in respiratory tract irritation, bronchitis, difficulty breathing, and stomach pains. Longer-term exposure can cause respiratory irritation, bronchial spasms, and coughing.
Regulatory limits
EPA – 50 parts per billion of selenium (50 ppb) in drinking water.
OSHA – 0.2 mg per cubic meter of workroom air for an 8-hour work shift.
Silver
Silver usually combines with other elements such as sulfide, chloride, and nitrate. Silver is used to make jewelery, silverware, electronic equipment, and dental fillings. Silver metal is also used in electrical contacts and conductors, in brazing alloys and solders, and in mirrors. Silver compounds are used in photographic film. Dilute solutions of silver nitrate and other silver compounds are used as disinfectants and as an antibacterial agent. Silver iodide has been used in attempts to seed clouds to produce rain.
Health effects:
Exposure to high levels for a long period may result in a condition called arygria, a blue- gray discoloration of the skin and other body tissues. Argyria appears to be a cosmetic problem that may not be otherwise harmful to health. Exposure to high levels of silver in the air has resulted in breathing problems, lung and throat irritation, and stomach pains. Skin contact with silver can cause mild allergic reactions such as rash, swelling, and inflammation in some people.
Regulatory limits:
EPA – recommends concentration in drinking water not to exceed 0.10 parts per billion (ppb). Requires that spills or accidental releases of 1,000 pounds or more be reported.
OSHA – in workplace air, 0.01 milligrams per cubic meter (0.01 mg/m³) for an 8-hour workday, 40-hour workweek.
Concerns about heavy metals:
The Dangerous Substances Directive of the European Union (76/464/EEC) defines dangerous chemicals as those which are toxic, persistent and / or bioaccumulative. As they are elements, they cannot be broken down; therefore heavy metals will persist in the environment. Unlike many organic pollutants, which eventually degrade to carbon dioxide and water, heavy metals will tend to accumulate in the environment, especially in lake, estuarine or marine sediments. Metals can be transported from one environment compartment to another.
Whether the source of heavy metals is natural or anthropogenic, the concentrations in terrestrial and aquatic organisms are determined by the size of the source and adsorption/precipitation in soils and sediments. The extent of adsorption depends on the metal, the absorbent, the physio-chemical characteristics of the environment (e.g. pH, water hardness and redox potential) and the concentrations of other metals and complex chemicals present.
Critical levels and loads:
Most critical levels derive from the European Union's Dangerous Substances Directive (76/464/EEC) and the US EPA (US EPA 1998) for water and the EU Air Quality Framework Directive (96/62/EEC) and the World Health Organization (World Health
Organization 1987) for air. The first Air Quality Daughter Directive sets critical levels for lead and another in preparation will sets limits for cadmium, arsenic, nickel and mercury. The general aim of the Air Framework and Daughter Directives is to avoid, prevent and reduce harmful effects of air pollutants on human health and the environmental as a whole. The National Air Quality Strategy is the means by which this European Union legislation is being implemented in the United Kingdom. The EU sludge to land directive (86/278/EEC) sets critical levels for heavy metals in agricultural soils that receive sewage sludge.
The UK is obliged under the Dangerous Substances Directive to set Environmental Quality Standards (EQSs; critical levels) for selected (List I and II) chemicals in water which are of national concern and the Environmental Protection Act 1990 requires EQSs for further chemicals (the Red List) in order to protect the aquatic environment. Critical levels for mercury and cadmium are available and one for arsenic is imminent.
Conclusions:
The EEC Directives clearly state the requirements for the quality of shellfish, and heavy metals are mentioned in this 1979 Directive. (See Appendix 4).
It states, inter alia, that the concentration of each heavy metal in shellfish flesh must be so limited that it contributes in accordance with Article 1, to the high quality of shellfish products
Article 1:
This Directive concerns the quality of shellfish waters and applies to those coastal and brackish waters designated by the Member States as needing protection or improvement in order to support shellfish (bivalve and gasteropod molluscs) life and growth and thus to contribute to the high quality of shellfish products directly edible by man.
In able to ascertain a greater understanding and put together a comprehensive analysis of the situation, we would need to be able to access various data from States Departments to compile background information. There appears to be difficulties in obtaining information from the various departments and there is only limited data which is available to the public for viewing.
A full and comprehensive risk analysis needs to be undertaken as soon as possible to limit any further damage to the marine environment and in turn public health.
A comprehensive monitoring program needs to be independently established as soon as possible. Save Our Shoreline does not own the necessary monitoring equipment to be able to do this, however all results could be interpreted and collated to provide a full analysis of the situation.
Example of factors to be taken into account during monitoring:
- Scope of monitoring – sediment, soil, leachate, biological indicators, sea water samples (ideally from St Aubins, Elizabeth Castle to Gorey at specified intervals), testing of various shellfish/ fish/ lobsters for heavy metals which are for sale to the public, aquatic plants etc.
- Preparation of list of key pollutants to test for
- Tidal flows, weather, season, sewage outflows, other pollution incidents etc.
Perhaps one of the most important aspects to analyse is the sediment. The sediment is the deposit of silt and accumulated organic and /or inorganic materials at the bottom of rivers, lakes, seas, etc. sediments can act as sinks for pesticides and heavy metals and can contain concentrations as much as 800 times that of water in the case of dieldrin (one of the group of chlorinated hydrocarbons). Chlorinated hydrocarbons are one of the three major groups of synthetic insecticides. This group includes endosulfan which is highly toxic to fish. In general, in fishes they have the effect of preventing oxygen uptake, causing suffocation. These hydrocarbons are part of the organochlorines group, which are characterized by their persistence, mobility and high biological activity. They have very long half lives and have a high capacity to injure living systems and allied with their other attributes may possibly constitute the greatest threat to our life-supporting ecosystems and associated biological cycles. The deposits of sediment are therefore a source of secondary contamination as well as allowing the bottom feeders to accumulate the pollutants at much greater concentrations than water analysis would indicate, thereby contaminating the food chain. As we have strong undercurrents in places it would be entirely feasible that sections of highly polluted sediment could be moved around the bay having almost a tidal effect on the sea-bed, therefore circulating the problem' around.
The problem is complicated and there are no easy answers, but it is possible to control NPS pollution and nature will respond to restoration efforts. The best way to solve our NPS pollution problems is through effective local government and individual citizen intervention.
An example of pollution potential:
 One gallon of used motor oil can pollute up to 2 million gallons of water.
One gallon of used motor oil can pollute up to 2 million gallons of water.
End note:
Not all possible pollutants to the marine environment have been covered in this report.
APPENDIX 1
![]() The relationship between the standard of the EIS and the Planning and Building (Jersey) Law 2002
The relationship between the standard of the EIS and the Planning and Building (Jersey) Law 2002
Note: (In English law it is referred to as an Environmental Impact Assessment with the non-technical summary known as the Environmental Impact Statement – in can be found in the Building Regulations 2005)
In Jersey law it is referred to as an Environmental Impact Statement with the non- technical summary known as the Environmental Statement.)
The EIS comes under Article 13 of the Planning and Building (Jersey) Law.
The Planning and Building (Environmental Impact) (Jersey) Order 2006, is in pursuance of Article 13 of the Planning and Building (Jersey) Law 2002.
In other words this Order is an extension of Article 13.
The Planning and Building (Environmental Impact) (Jersey) Order 2006
The incinerator comes under the section 11(4) Schedule 1, that requires an EIS by law and has to meet the criteria of Schedule 2.
(1) Except as provided by paragraph (2), proposed development specified in column 1 of Schedule 1 is prescribed development for the purpose of Article 13(1)(a) of the Law.
![]() SCHEDULE 1
SCHEDULE 1
DESCRIPTIONS OF DEVELOPMENT IN RESPECT OF WHICH AN ENVIRONMENTAL IMPACT STATEMENT IS REQUIRED
11 Other projects
(4) The construction of an installation for the disposal or treatment of waste (including waste from mines and quarries and vegetable waste).
Therefore all aspects of the EIS have to be met by Law.
WHAT AN ENVIRONMENTAL IMPACT STATEMENT MUST CONTAIN
PART 1
- A description of the aspects of the environment likely tobe significantly
affected by the development, including, in particular, population, fauna, flora, soil, water, air, climatic factors, material assets, including the architectural and archaeological heritage, landscape and the inter-relationship between the above factors.
- A description of the likely significant effects of the development on the environment, which should cover the direct effects and any indirect, secondary, cumulative, short, medium and long-term, permanent and temporary, positive and negative effects of the development, resulting from –
- the existence of the development;
- the use of natural resources;
- the emission of pollutants, the creation of nuisances and the elimination of waste,
and the description by the applicant of the forecasting methods used to assess the effects on the environment.
- A description of the measures envisaged to prevent, reduce and where possible offset any significant adverse effects on the environment.
There has been a failure, by omission in Articles 3, 4 and 5 where no inter-relationship has been assessed between factors. Ramsar – Air pollutants, also leaching through ground soil to sea – from waste storage etc. As a result of the absence of this information, no mitigation has been put into place for environmental and human health in the EIS.
Under Article 13 (3) of the Planning and Building (Jersey) Law 2002, the Minister has to take the statement into account in determining the application.
13 Environmental impact of proposed development
(3) The Minister shall take the statement into account in determining the application.
Please note the part about a representation with a material omission below, with reference to the omission above under Articles 3, 4 and 5.
PART 3
![]() PLANNING CONTROL
PLANNING CONTROL
10 False information, etc. in application for planning permission
- If when making an application for planning permission a person knowingly or recklessly makes a false or misleading statement or representation or a statement or representation with a material omission
The following remedial action can be taken by the Minister for a representation with material omission – revoking the planning permission if it has already been granted.
- If a person has made such a statement or representation and the planning permission has been granted, the Committee may –
(a) revoke or modify the permission
Relevant parts of the Laws:
Planning and Building (Jersey) Law 2002
PART 3
![]() PLANNING CONTROL
PLANNING CONTROL
10 False information, etc. in application for planning permission
- If when making an application for planning permission a person knowingly or recklessly makes a false or misleading statement or representation or a statement or representation with a material omission the person shall be guilty of an offence and liable to imprisonment for a term of 2 years and a fine.[7]
- If a person has made such a statement or representation and the planning permission has been granted, the Committee may –
- revoke or modify the permission; and
- if the development has been started or undertaken, serve a notice on the owner of the land to which the permission relates.
- The notice may require the owner of the land, within a period specified in the notice –
- to undertake work specified in the notice to restore the land to its condition before the development was undertaken; or
- to modify the development to the extent specified in the notice.
- The work tobe undertaken may include –
- the demolition or alteration of the whole or anypart of a building; or
- the discontinuance of a use of land.
- The Minister may act in accordance with paragraph (2) whether or not proceedings have been taken in respect of the offence under paragraph (1).
- A person who –
- fails to comply with a notice served on the person in accordance with paragraph (2)(b); or
- uses land in contravention of the notice,
shall be guilty of an offence and liable to a fine of level 3 on the standard scale.
- If at the end of the period for compliance specified in a notice under paragraph (2)(b), work required bythe notice to be undertaken has not been undertaken, the Minister may enter the land and undertake the work.
- The expenses reasonably incurred by the Minister in undertaking work in accordance with paragraph (7) shall be recoverable as a debt due to the Minister from the person in default.
- The Minister may undertake work in accordance with paragraph (7) whether or not proceedings have been taken under paragraph (6).
- Action taken bythe Minister under this Article does not give any person the right to claim compensation in respect of any loss or damage the person may suffer as a result of the action.
13 Environmental impact of proposed development
- This Article applies in respect of an application for planning permission –
- to carry out development that falls within a class of development prescribed for the purpose of this sub-paragraph; or
- where the Minister is satisfied that if the proposed development were to be carried out it would be likely to have a significant effect on the environment of Jersey or elsewhere.
- Where this Article applies the Minister shall not consider the application until the applicant has provided the Minister with an environmental impact statement.
- The Minister shall take the statement into account in determining the application.
- The Minister shall by Order prescribe for the purpose of paragraph (1)(a) classes of development in respect of which an environmental impact statement is required.
- The Order shall also prescribe –
- the particulars an environmental impact statement must contain;
- the qualifications of the people by whom those particulars are to be provided;
- the form an environmental impact statement isto take; and
- such other matters as the Minister considers relevant to the preparation and provision of an environmental impact statement.
16 Development of concern to the Minister for Transport and Technical Services
- This Article applies in respect of an application for planning permission for development that falls within an area of responsibility or concern of the Minister for Transport and Technical Services.
- Where this Article applies the Minister shall refer the application to the Minister for Transport and Technical Services for comment.
- The Minister shall in determining the application take into account any comment made bythe Minister for Transport and Technical Services in respect of the matters specified in paragraph (4).
- Those matters are –
- the sufficiency of any sewerage or drainage system, flood defence work or water course that may be affected bythe development, the prevention of damage to it, and any hindrance to its repair or maintenance;
- the limitation of damage by surface water that could be caused bythe development;
- the effect of the development on water quality (including sea water quality).
Planning and Building (Environmental Impact) (Jersey) Order 2006
2 Prescribed development requiring an environment impact statement
- Except as provided by paragraph (2), proposed development specified in column 1 of Schedule 1 is prescribed development for the purpose of Article 13(1)(a) of the Law.
- If a qualifying criterion is specified in column 2 in respect of any proposed development, it is not prescribed development for the purpose of Article 13(1)(a) of the Law unless it meets that criterion or, if more than one criterion is specified in column 2, at least one such criterion.
- Where proposed development mentioned in paragraph (1) is already authorised, executed or inthe process of being executed, a change or extension of the development is also prescribed development for the purpose of Article 13(1)(a) of the Law.
- Development mentioned in paragraph (1) or paragraph (3) is not prescribed development for the purpose of Article 13(1)(a) of the Law if the Minister is satisfied that by virtue of factors such as the nature, size or location
of the proposed development it would be unlikely, if carried out, to have a |
significant effect on the environment, either of Jersey or elsewhere. |
|
|
|
|
|
|
|
SCHEDULE 1 |
|
DESCRIPTIONS OF DEVELOPMENT IN RESPECT OF WHICH AN |
ENVIRONMENTAL IMPACT STATEMENT IS REQUIRED |
|
|
11 Other projects |
(4) The construction of an installation for the disposal or treatment of waste (including waste from mines and quarries and vegetable waste).
 The change of use of land to use as a site for the storage of scrap metal.The land
The change of use of land to use as a site for the storage of scrap metal.The land
is within 100 metres of a stream, pond or reservoir.
SCHEDULE 2 (Article 1)
WHAT AN ENVIRONMENTAL IMPACT STATEMENT MUST CONTAIN
PART 1
- Description of the development, including in particular –
- a description of the physical characteristics of the whole development and the land-use requirements during the construction and operational phases;
- a description of the main characteristics of the production processes, for instance, nature and quantity of the materials used;
- an estimate, by type and quantity, of expected residues and emissions (water, air and soil pollution, noise, vibration, light, heat, radiation, etc.) resulting from the operation of the proposed development.
- An outline of the main alternatives studied by the applicant and an indication of the main reasons for his or her choice, taking into account the environmental effects.
- A description of the aspects of the environment likely to be significantly affected by the development, including, in particular, population, fauna, flora, soil, water, air, climatic factors, material assets, including the architectural and archaeological heritage, landscape and the inter-relationship between the above factors.
- A description of the likely significant effects of the development on the environment, which should cover the direct effects and any indirect, secondary, cumulative, short, medium and long-term, permanent and temporary, positive and negative effects of the development, resulting from –
- the existence of the development;
- the use of natural resources;
- the emission of pollutants, the creation of nuisances and the elimination of waste,
and the description by the applicant of the forecasting methods used to assess the effects on the environment.
- A description of the measures envisaged to prevent, reduce and where possible offset any significant adverse effects on the environment.
- A non-technical summary of the information provided under paragraphs 1 to 5 of this Part.
- An indication ofany difficulties (technical deficiencies or lack of know- how) encountered bythe applicant in compiling the required information.
PART 2
- A description of the development comprising information on the site, design and size of the development.
- A description of the measures envisaged in order to avoid, reduce and, if possible, remedy significant adverse effects.
- The data required to identify and assess the main effects which the development is likely to have on the environment.
- An outline of the main alternatives studied by the applicant and an indication of the main reasons for his or her choice, taking into account the environmental effects.
- A non-technical summary of the information provided under paragraphs 1 to 4 of this Part
APPENDIX 2








Appendix 3




Appendix 4:






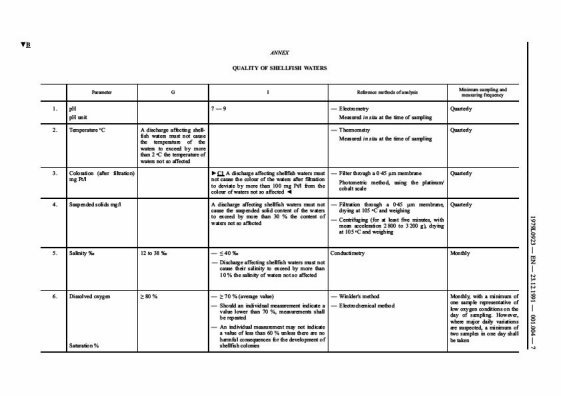
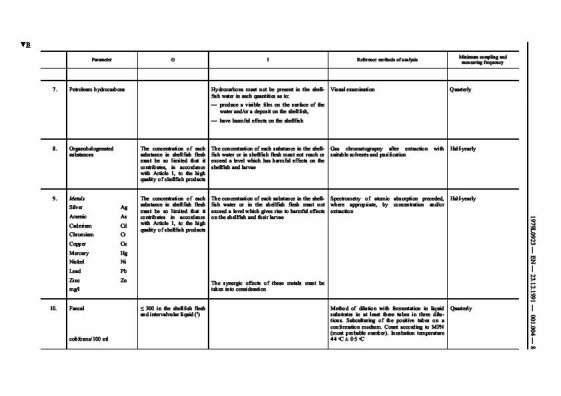
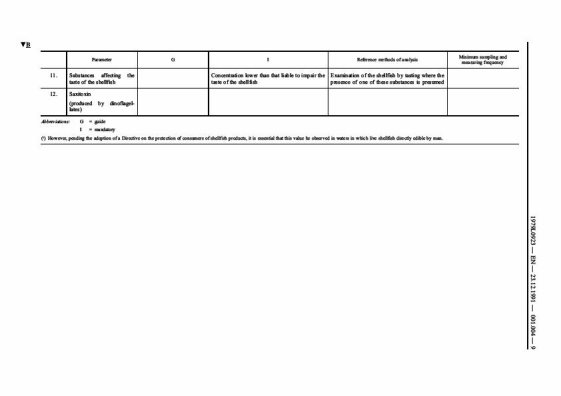
References:
IWA Publishing, Basic Water Treatment (3rd Edition), Chris Binnie, Martin Kimber and George Smethurst.
Porteous, A. (2000) Dictionary of Environmental Science and Technology, 3rd edition, London, John Wiley and Sons.
Open University (2006), T308 Block, Potable Water Treatment, Milton Keynes, The Open University.
Publish America Baltimore (2007), Heavy Metals, Art Smith
The Royal Society of Chemistry (1990), Pollution, Causes Effects and Controls, (2nd Edition), Roy M Harris on
The Royal Society of Chemistry (2001), 16 Issues in Environmental Science and Technology, Assessment and Reclamation of Contaminated Land, R. E. Hester and R. M. Harris on
Pergamon Press Ltd (1973), Progress in Water Technology, Volume 3, (collection of authors – consultation papers)
